Voices of Biotech
Podcast: MilliporeSigma says education vital to creating unbreakable chain for sustainability
MilliporeSigma discusses the importance of people, education, and the benefits of embracing discomfort to bolster sustainability efforts.
February 13, 2017
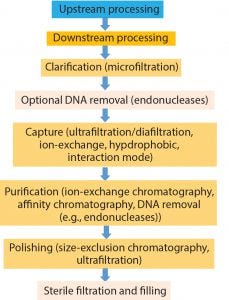
Figure 1: Viral vector production
Gene therapy is the transfer of genetic material to a patient’s cells to achieve a therapeutic effect. Therapeutic DNA is largely delivered using viral vector systems based on adenoviruses (Ad), adenoassociated viruses (AAV), and lentiviruses (LV). With the application of such viral vectors as clinical therapeutics growing, scalable commercial processes (particularly for purification) are being investigated and optimized to best ensure that critical quality attributes (CQAs) are retained. Herein we review viral vector purification techniques and the effect of different characteristics of vector classes on the selection of optimal unit operations.
View the full article below – Login Required
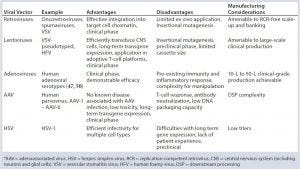
Table 1: Viral vector overview (46–50)
Viral Vector Classes
Each class of viral vector has its own challenges and opportunities for gene therapeutics (Table 1). Adenoviruses were initially proposed for gene therapy over 20 years ago, but they are now less popular for clinical applications because of significant complexities such as their challenging vector design and systematic administration, large genome size, and (most important) their high immunogenicity (1).
Lentiviruses already have shown promise in clinical trials, for which they have been used in the treatment of Wiskott–Aldrich syndrome, X-linked adrenoleukodystrophy, and metachromatic leukodystrophy (2–4). The central mechanisam of action (MoA) for lentiviruses relates to their ability to transduce both dividing and nondividing cells, resulting in life-long transgene expression and potential therapeutic effect.
AAVs also are considered a promising tool for gene therapy because of their lack of pathogenicity and immunogenicity paired with long-term transgene expression and broad cell tropism (5). Currently, the only commercialized gene therapy in the Western world is Glybera (alipogene tiparvovec) from uniQure, which is based on AAV1 and has been used to treat hereditary lipoprotein lipase deficiency (LPLD).
Biomanufacturing Considerations
Gene therapy applications require large-scale processes that can generate highly pure and biologically active vectors that fulfill regulatory chemistry, manufacturing, and controls (CMC) requirements. They should be robust, scalable, cost-effective, and ideally applicable to a large variety of viral vectors (6). As Figure 1 shows, viral vector production consists of upstream and downstream processes comprising several steps, depending on the viral properties of a product.
Upstream processing of virus particles for gene therapy vectors entails virus growth and harvesting, whereas downstream processing is focused at viral vector purification. Notably, downstream purification of viral vectors accounts for the majority of the overall manufacturing cost, and it represents a bottleneck in viral vector manufacturing (7). Scalable downstream processing contains several steps: clarification (microfiltration), capture (ultrafiltration/diafiltration), purification (ion-exchange chromatography (IEX) and affinity chromatography (AF)), and polishing (size-exclusion chromatography (SEC) and ultrafiltration).
Because each virus has different biochemical and physical properties, viral gene-therapy vector purification must be tailored accordingly. This process requires optimization to preserve virus infectivity (closely related to product efficacy and a typical release assay) and maximize recovery. The decision regarding purification method should take characteristics such as virus particle size and stability, charge at neutral pH, and relative particle stability into consideration (8). You must thoroughly understand your purification process to identify critical steps that affect final-product quality.
Viral Vector Purification Methods
Several approaches can be taken for viral gene therapy vector purification, including ultracentrifugation and membrane and column chromatography methods. The latter two are amenable to scale-up.
Ultracentrifugation is used mainly at laboratory scale and is not scalable because available rotors usually have only small-volume capacities. It frequently results in a loss of active viral particles, which could be attributed to viral aggregation and shear forces among other possible explanations (8). Compared with membrane and column chromatography, ultracentrifugation is challenging to automate. Its use can increase processing times and the risk of product degradation.
Although column chromatography tools are scalable and routinely used for purifying biomolecules, they are not well suited for purification of larger molecules such as DNA and viruses. In such cases, purification typically is achieved by diffusion through a 0.1-µm matrix.
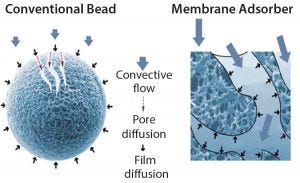
Figure 2: Conventional chromatography compared with membrane adsorbers
With membrane pores >3 µm, membrane adsorbers have become popular tools for purifying viruses. They adsorb virus particles to a solid phase. Because of their large pore size, membrane adsorbers can be operated at significantly faster flow rates, therein resulting in significant time and cost of goods (CoG) savings (9). Another advantage is their possibility of applying mild elution conditions, which increases the likelihood of preserving virus infectivity (6). Figure 2 illustrates how virus purification is achieved with membrane adsorbers as opposed to using beads.
Modes of Purification Chromatography
Chromatography can be divided further into four modes, each achieving virus purification by using a different mechanism. Purification through IEX is based on the net charge of proteins located on the outside of the viral capsid. AF exploits interactions between a matrix-coupled receptor or ligand with the viral capsid. SEC (gel filtration) separates viruses from DNA, capsids, and proteins according to size. And hydrophobic-interaction chromatography (HIC) binds viral capsid proteins to a matrix through hydrophobic interaction using an aqueous solvent (10).
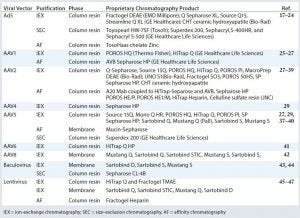
Table 2: Vector purification chromatography products
Modes of Choice Based on Vector Class
Because each vector class has specific characteristics, certain purification modes are apt to function more effectively (Table 2). For the purification of adenoviruses, namely Ad5, all aforementioned chromatographic methods can be used. Because Ad viruses are negatively charged at a neutral pH, various anion-exchange adsorbents can be used for their purification. IEX using anion exchangers also has been reported for several AAVs (11). Immobilized-metal affinity chromatography (IMAC) is another method by which Ad can be purified. That approach is based on binding Ad particles to charged zinc ions bound to a column. That method provides several options for purifying AAVs, exemplified by heparin affinity chromatography, which is particularly suited for AAV2 purification (12). SEC is a suitable method for polishing Ad and AAV5, and HIC purifies Ad particles with high concentrations of ammonium sulfate among other salts (13). The latter method has yet to be described for the purification of AAV serotyes (10).
Because of their negative charge, lentiviruses are routinely purified using membrane adsorbers and AEX tools based on columns used in a capture step. This step varies the most among different viral vector purification groups. Lentiviruses also can be purified by using heparin affinity chromatography, but it introduces an animal-derived reagent that should be elminated in large-scale gene-therapy manufacturing (14). Finally, baculoviruses are commonly purified using membrane-based anion exchangers in the capture step, anion-exchange membrane adsorbers, and SEC.
Progress, But Work Remains
The selection of purification technologies in gene therapy bioprocessing is critical to end-product quality and CoG and very much a multifactorial process. Although platform bioprocesses are emerging for gene therapy bioprocessing, they are not yet established as such. Each vector requires thorough characterization and process development. However, classical large-scale purification technologies (particularly chromatographic approaches) are highly amenable to gene therapy bioprocessing. As such, this emerging industry would be well served to seek to unite emerging basic science expertise in genetic engineering approaches with established bioprocessing skills and technologies.
Tools and technologies required to build a sustainable and profitable gene-therapy industry are within our grasp. But the challenge at hand is determining how to configure them optimally to accelerate development of life-saving and efficacious gene therapies to the clinic, with data packages that pass regulatory muster and a cost that providers can afford.
References
1 Lukashev AN, Zamyatnin AA Jr. Viral Vectors for Gene Therapy: Current State and Clinical Perspectives. Biochemistry (Mosc). 81(7) 2016: 700–708; doi: 10.1134/S0006297916070063.
2 Aiuti A, et al. Lentiviral Hematopoietic Stem Cell Gene Therapy in Patients with Wiskott–Aldrich Syndrome. Science 341(6148) 2013: 1233151; doi: 10.1126/science.1233151.
3 Cartier N, et al. Hematopoietic Stem Cell Gene Therapy with a Lentiviral Vector in X-Linked Adrenoleukodystrophy. Science 326(5954) 2009: 818–823; doi: 10.1126/science.1171242.
4 Biffi A, et al. Lentiviral Hematopoietic Stem Cell Gene Therapy Benefits Metachromatic Leukodystrophy. Science 341(6148) 2013: 1233158; doi: 10.1126/science.1233158.
5 dos Santos Coura R, Beyer Nardi N. Genetics A Role for Adenoassociated Viral Vectors in Gene Therapy. Genetics Mol. Bio. 31(1) 2008: 1–11.
6 Morenweiser R. Downstream Processing of Viral Vectors and Vaccines. Gene Ther. Oct. 2005: S103–S110.
7 Lyddiatt A, O’Sullivan D. Biochemical Recovery and Purification of Gene Therapy Vectors. Curr. Opin. Biotechnol. 9(2) 1998: 177–185.
8 Segura MM, Kamen A, Garnier A. Downstream Processing of Oncoretroviral and Lentiviral Gene Therapy Vectors. Biotechnol. Adv. 24(3) 2006: 321–337.
9 Ball PR, Gyepi-Garbrah I. Membrane Adsorbers as a Tool for Rapid Purification of Gene Therapy Products.
10 Burova E, Ioffe E. Chromatographic Purification of Recombinant Adenoviral and Adenoassociated Viral Vectors: Methods and Implications. Gene Ther. Oct. 2005: S5–S17.
11 Gao G, Vandenberghe LH, Wilson JM. New Recombinant Serotypes of AAV Vectors. Curr. Gene Ther. 5(3) 2005: 285–297.
12 Clark K, et al. Highly Purified Recombinant AdenoAssociated Virus Vectors are Biologically Active and Free of Detectable Helper and Wild-Type Viruses. Hum. Gene Ther. 10(6) 1999: 1031–1039.
13 Huyghe BG, et al. Purification of a Type 5 Recombinant Adenovirus Encoding Human p53 By Column Chromatography. Hum. Gene Ther. 6(11) 1995: 1403–1416.
14 Merten OW, Hebben M, Bovolenta C. Production of Lentiviral Vectors. Mol. Ther. Methods Clin. Dev. 13 (3) 2016: 16017. doi: 10.1038/mtm.2016.17.
15 Lusky M. Good Manufacturing Practice Production of Adenoviral Vectors for Clinical Trials. Hum. Gene. Ther. 16, 2005: 281–291.
16 Green AP, et al. A New Scalable Method for the Purification of Recombinant Adenovirus Vectors. Hum. Gene Ther. 13(16) 2002: 1921–1934.
17 Kamen A, Henry O. Development and Optimization of an Adenovirus Production Process. J. Gene Med. 6, 2004: S184–192.
18 Huyghe BG, et al. Purification of a Type 5 Recombinant Adenovirus Encoding Human p53 By Column Chromatography. Hum. Gene Ther. 6(11), 1995: 1403–1416.
19 Blanche F, et al. An Improved Anion-Exchange HPLC Method for the Detection and Purification of Adenoviral Particles. Gene Ther. 7(12) 2000: 1055–1062.
20 Peixoto C, et al. Purification of Adenoviral Vectors Using Expanded-Bed Chromatography. J. Virol. Methods 132(1–2) 2006: 121–126.
21 Vellekamp G, et al. Empty Capsids in ColumnPurified Recombinant Adenovirus Preparations. Hum. Gene Ther. 12(15) 2001: 1923–1936.
22 Konz JO, et al. Development of a Purification Process for Adenovirus: Controlling Virus Aggregation to Improve the Clearance of Host Cell DNA. Biotechnol. Prog. 21, 2005: 466–472.
23 Smith R H, Levy JR, Kotin RM. A Simplified Baculovirus-AAV Expression Vector System Coupled with One-Step Affinity Purification Yields High-Titer rAAV Stocks from Insect Cells. Mol. Ther. 17(11) 2009: 1888–1896; doi: 10.1038/mt.2009.128.
24 Urabe M, et al. Removal of Empty Capsids from Type 1 Adeno-Associated Virus Vector Stocks By Anion-Exchange Chromatography Potentiates Transgene Expression. Mol. Ther. 13(4) 2006: 823–828.
25 Zolotukhin S, et al. Production and Purification of Serotype 1, 2, and 5 Recombinant Adenoassociated Viral Vectors. Methods 28(2) 2002: 158–167.
26 Qu G, et al. Separation of Adeno-Associated Virus Type 2 Empty Particles from Genome Containing Vectors By Anion-Exchange Column Chromatography. J. Virol. Methods 140(1–2) 2007: 183–192.
27 Kaludov N, Handelman B, Chiorini JA. Scalable Purification of Adeno-Associated Virus Type 2, 4, or 5 Using Ion-Exchange Chromatography. Hum. Gene Ther. 13, 2002: 1235–1243.
28 Gao G, et al. Purification of Recombinant Adenoassociated Virus Vectors By Column Chromatography and Its Performance In Vivo. Hum. Gene Ther. 11(15) 2000: 2079–2091.
29 O’Riordan, et al. Scalable Chromatographic Purification Process for Recombinant Adeno-Associated Virus (rAAV). J. Gene Med. 2, 2000: 444–454.
30 Brument N, et al. A Versatile and Scalable Two-Step Ion-Exchange Chromatography Process for the Purification of Recombinant Adeno-Associated Virus Serotypes-2 and -5, Mol. Ther. 6(5) 2002: 678–686.
31 Zolotukhin S, et al. Recombinant Adenoassociated Virus Purification Using Novel Methods Improves Infectious Titer and Yield. Gene Ther. 6(6) 1999: 973–985.
32 Chahal PS, Aucoin MG, and Kamen A. Primary Recovery and Chromatographic Purification of Adenoassociated Virus Type 2 Produced By Baculovirus/Insect Cell System. J. Virol. Methods 139, 2007: 61–70.
33 Grimm D, et al. Novel Tools for Production and Purification of Recombinant Adenoassociated Virus Vectors, Hum. Gene Ther. 9, 1998: 2745–2760.
34 Clark KR, et al. Highly Purified Recombinant Adenoassociated Virus Vectors Are Biologically Active and Free of Detectable Helper and Wild-Type Viruses. Hum. Gene Ther. 10, 1999: 1031–1039.
35 Anderson R, et al. A Method for the Preparation of Highly Purified Adenoassociated Virus Using Affinity Column Chromatography, Protease Digestion and Solvent Extraction. J. Virol. Methods 85, 2000: 23–34.
36 Harris JD, Beattie SG, Dickson JG. Novel Tools for Production and Purification of Recombinant Adenoassociated Viral Vectors. Methods Mol. Med. 76, 2003: 255–267.
37 Smith RH, Ding C, Kotin RM. Serum-Free Production and Column Purification of Adenoassociated Virus Type 5. J. Virol. Methods 114, 2003: 115–124.
38 Auricchio A, et al. A Single-Step Affinity Column for Purification of Serotype-5–Based Adenoassociated Viral Vectors. Mol. Ther. 4, 2001: 372–374.
39 Sayroo R, et al. Development of Novel AAV Serotype 6 based Vectors with Selective Tropism for Human Cancer Cells. Gene Ther. 1, 2016: 18–25.
40 Davidoff AM. Purification of Recombinant AdenoAssociated Virus Type 8 Vectors By Ion-Exchange Chromatography Generates Clinical Grade Vectorstock. J. Virol. Methods 121, 2004: 209–215.
41 Vicente T, et al. Purification of Recombinant Baculoviruses for Gene Therapy Using Membrane Processes, Gene Ther. 16, 2009: 766–775.
42 Wu C, Soh KY, Wang S. Ion-Exchange Membrane Chromatography Method for Rapid and Efficient Purification of Recombinant Baculovirus and Baculovirus gp64 Protein. Hum. Gene Ther. 18, 2007: 665–672.
43 Scherr M. Efficient Gene Transfer into the CNS By Lentiviral Vectors Purified By Anion Exchange Chromatography. Gene Ther. 9, 2002: 1708–1714.
44 Yamada K, et al. Lentivirus Vector Purification Using Anion Exchange HPLC Leads to Improved Gene Transfer. Biotechniques 34, 2003: 1074–1078, 1080.
45 Segura MM, et al. Production of Lentiviral Vectors By Large-Scale Transient Transfection of Suspension Cultures and Affinity Chromatography Purification. Biotechnol. Bioeng. 98, 2007: 789–799.
46 Pettitt D, et al. Emerging Platform Bioprocesses for Viral Vectors and Gene Therapies. BioProcess Int. 14(4) 2016: S8–S17.
47 Rincon MY, Vandendriessche T, Chuah MK. Gene Therapy for Cardiovascular Disease: Advances in Vector Development, Targeting, and Delivery for Clinical Translation. Cardiovas. Res. 108(1) 2015: 4–20.
48 Kay MA, Glorioso JC, Naldini L. Viral Vectors for Gene Therapy: The Art of Turning Infectious Agents into Vehicles of Therapeutics. Nature Med. 7(1) 2001: 33–40.
49 Sheu J, et al. Large-Scale Production of Lentiviral Vector in a Closed System Hollow Fiber Bioreactor. Mol. Ther. Meth. Clin. Dev. 2, 2015: 15020.
50 Kallel H, Kamen AA. Large‐Scale Adenovirus and Poxvirus‐Vectored Vaccine Manufacturing to Enable Clinical Trials. Biotechnol. J. 10(5) 2015: 741–747.
Maya Fuerstenau-Sharp is a field-marketing manager at Sartorius Stedim (Göttingen, Germany). David Pettitt is an academic surgeon, CASMI research associate, associate at IP Asset Ventures, Ltd, and a doctoral researcher at the University of Oxford. Kim Bure is director of regenerative medicine at Sartorius Stedim. James Smith is a CASMI research associate and doctoral researcher in the University of Oxford’s Nuffield Department of Orthopedics, Rheumatology, and Musculoskeletal Sciences, and an associate at IP Asset Ventures, Ltd. Jamie Ware is a CASMI research associate. Corresponding author David Brindley is founder and academic director of CASMI’s Translational Stem Cell Consortium, senior healthcare translation research fellow in the medical sciences division’s department of paediatrics at the University of Oxford, Cooksey-Saïd fellow in healthcare translation at the University of Oxford’s Saïd Business School, honorary senior research associate at the University College London School of Pharmacy’s Centre for Behavioral Medicine, a research fellow in cell therapy commercialization at the Harvard Stem Cell Institute, and regenerative medicine regulation and risk management lead at the USCF Stanford Center of Excellence in Regulatory Science and Innovation (CERSI) in Stanford, CA.
You May Also Like