Voices of Biotech
Podcast: MilliporeSigma says education vital to creating unbreakable chain for sustainability
MilliporeSigma discusses the importance of people, education, and the benefits of embracing discomfort to bolster sustainability efforts.

The nonionic surfactant Triton X-100 (C14H22O(C2H4O)n) is a key chemical used in ensuring the viral safety of biological medicinal products. Two pharmaceutical sectors share an extensive historical background with it: biopharmaceuticals and plasma-derived products, for which it is used to inactivate lipid-enveloped viruses. Recently, environmental regulations in the European Union have encouraged or mandated a phase-out of this surfactant (1). The goal of the ruling is to protect aquatic ecosystems from potential Triton X-100 degradation products that can function as endocrine disruptors with estrogen-mimetic activity. The phase-out will affect medicinal products for European markets, depending on approval status and the point of manufacture at which they enter the European Union. Below, we discuss the potential consequences of those regulations on both key industry sectors.
For Plasma-Derived Products
Solvent/detergent (S/D) inactivation is the industry-standard unit operation for ensuring viral safety of products derived from human blood plasma. To augment testing that could not provide 100% coverage because of a “window period,” the method was developed in the 1980s as part of the industry’s response to human immunodeficiency virus (HIV) contamination in the plasma supply. The idea was that the action of a detergent (e.g., cholate, Triton X-100, or polysorbate 80) and a solvent (typically tri(n-butyl)phosphate, TNBP) together could dissolve lipid envelopes around retroviruses such as HIV, rendering them inactive. This effort was pioneered by the New York Blood Center, where the first S/D treatment used a cholic acid salt (2). Subsequently, Baxter’s Hyland division developed what it called “method M,” which uses Triton X-100 treatment because of its perceived improved kinetics (3).
The widespread criticality of S/D recently was highlighted in a published data collection from 308 studies conducted by seven members of the Plasma Protein Therapeutics Association (PPTA) (4). The seven companies manufacture multiple plasma-derived products, including blood factors VIII and IX, intravenous immunoglobulin (IVIG), and intramuscular immunoglobulin (IMIG). Each product’s manufacturing scheme includes an S/D step in which either Triton X-100, cholate, or polysorbate 80 detergent is combined with TNBP solvent. The polysorbate is the most commonly used; Triton X-100 surfactant is second in use and often combined with polysorbate.
The PPTA members reported and evaluated the effectiveness of S/D in robustness studies and at a commercial set point based on data from their regulatory submissions. The companies used several model enveloped viruses for small-scale studies: pseudorabies virus (PRV), bovine viral diarrhea virus (BVDV), HIV-1, Sindbis virus (SINV), equine viral arteritis virus (EAV), West Nile virus (WNV), vesicular stomatitis virus (VSV), vaccinia virus (VacV), and severe acute respiratory syndrome coronavirus (SARS-CoV). The Triton X-100 surfactant proved to be effective for inactivating enveloped viruses, with the aggregated claims under production conditions as follows (4): between >2.9 and >6.5 log for the Triton X-100–TNBP combination; between >4.0 and >6.6 log for the same combination with polysorbate 80 added.
With it being the second most-used detergent for S/D unit operations by the plasma-product industry, clearly, phasing out or replacing Triton X-100–based S/D treatment would be disruptive. Existing processes using it are part of manufacturing many approved and marketed drugs. Although this change is not yet a legal regulatory requirement for existing products, it would require process revalidation efforts (including virus-inactivation studies with a panel of viruses) followed by a global regulatory-approval process. Furthermore, changing the S/D step would need to proceed without creating additional patient risk because S/D is a key safety measure. A suitable substitute would be required.
For Biopharmaceuticals
In the biopharmaceutical industry, the primary regulatory concern for a potential Triton X-100 phase-out is associated with enveloped retrovirus-like particles (RVLPs) produced by rodent-derived expression systems such as Chinese hamster ovary (CHO) cells. Those particles are mostly noninfectious, but internationally harmonized guidances mandate their clearance in downstream processing (5). Large numbers of RVLPs are produced during production, with up to 1010 particles/mL detectable in many clarified harvested cell culture fluids (HCCFs).
Figure 1: Retrovirus-like particles (RVLPs) common to many bioprocess host cell lines have lipid envelopes, so companies often use the closely related model, xenotropic murine leukemia virus (X-MuLV) for clearance studies to test inactivation methods such as Triton X-100 detergent treatment.
Fortunately, multiple steps in protein purification can remove or inactivate viruses: e.g., column chromatography, filtration, and low-pH incubation. Detergent treatment of HCCF has been proposed as a “fifth orthogonal step” (after two columns, low-pH inactivation, and virus filtration). Like retrovirus particles, RVLPs have lipid envelopes, so clearance studies can use a closely related model virus, xenotropic murine leukemia virus (X-MuLV, Figure 1). This fifth step could be helpful for products that need extra clearance of lipid-enveloped viruses, especially when one chromatography step doesn’t provide effective results or when production cells make high levels of RVLPs. For example, the mouse myeloma cell line SP2/0 is known to produce a high level of particles, a fraction of which can form foci in in vitro assays. X-MuLV inactivation studies have shown that nonionic surfactants are effective at inactivating murine retrovirus, even in HCCF (6).
To support such detergent-treatment applications, the American Society for Testing and Materials (ASTM) developed a standard (E3042-16) supporting claims of 4 log10 clearance of rodent retroviruses (e.g., MuLV and RVLPs) (7). The standard largely applies to monoclonal antibody (MAb) process fluids treated with Triton X-100 surfactant, and its scope includes a clarified, cell-free intermediate (0.2-µM filtered HCCF). That broader scope offers a great advantage over low-pH incubation unit operations, which can be applied only after capture chromatography. For process efficiency, after the S/D treatment, the subsequent capture column can remove the surfactant. That allows for a second inactivation step (e.g., low pH) to be applied to more-purified intermediates further downstream.
Triton X-100 inactivation of rodent retrovirus ASTM standard is carried out with the following parameters: Triton X-100 concentration ≥0.5%, time ≥60 min (with mixing confirmed), pH 6.0–8.0, and temperature 15–25 °C (room temperature according to harmonized guidelines).
EU Environmental Legislation
The EU Registration, Evaluation, Authorization and Restriction of Chemicals (REACH) Regulation of 2006 entered into force in June 2007 (1). Its aim is to provide oversight within the European Union over potentially harmful chemicals. To that end, it addresses the production and use of chemicals and their potential impacts on both human health and the environment. The regulation also applies to chemicals found in goods, so it can affect importation of relevant substances into Europe either on their own or as part of imported goods.
The technical aspects of the REACH rules are implemented and managed by the European Chemicals Agency (ECHA), which is based in Helsinki, Finland. That agency has set up a registration system for chemicals used within the European Union, and by early 2022 nearly 23,000 substances had been entered into its database (8). The legislation requires companies to register information about the hazards, risks, and safe use of chemicals that they manufacture or import with the ECHA. Such information then gets published on the agency’s website.
The existing global classification and labeling system for chemicals makes it easy to identify hazards associated with specific substances under EU regulation 1907/2006. Hazardous substances are classified into a number of categories. Then, substances of very high concern (SVHC) are controlled, making their use subject to authorization. The ultimate goal is to replace them progressively with less dangerous substances.
SVHCs include substances that are carcinogenic, mutagenic, and/or toxic to reproductive systems. For environmental concerns, those include substances that are persistent, bioaccumulative, and toxic (PBT substances) and those that are “very persistent and very bioaccumulative” (known as vPvB substances). According to the REACH legislation, such classification requires “scientific evidence of probable serious effects to human health or the environment which give rise to and equivalent level of concern.” That is decided case by case.
Procedurally, substances first get placed on a candidate list following initiation from a member state and after a short consultation period. Such listing brings a number of obligations to suppliers of candidate substances for providing information to customers and consumers. The ECHA reviews its candidate list and prioritizes substances, then moves some to an authorization list after further consultation. Once a substance is on the authorization list, it can be used only for nonexempted applications if authorized. Normally, a deadline is set after which no further authorization applications will be accepted.
Triton X-100 Impacts
Triton X-100 surfactant is part of a group of chemicals known as octylphenol ethoxylates, which have toxic effects on aquatic organisms even at low concentrations (9). It was added to the ECHA candidate list in December 2012 as part of the substance group 4-(1,1,3,3-tetramethylbutyl) phenol, ethoxylated. That addition was based on concerns about the environmental impact of nonethoxylated degradation products of these substances acting as endocrine disruptors with estrogen-mimetic activity, an effect that is particularly harmful to aquatic animals. The degradation product 4-(1,1,3,3-tetramethylbutyl)phenol thus is considered to be both an SVHC and a PBT (10).
In 2017, the surfactant was included in the ECHA’s authorization list (Annex XIV) as an SVHC (11). Triton X-100 manufacturers had an opportunity to apply for authorization to continue using the compound past the sunset period. But the time to apply for exemptions has now passed, and Triton X-100 use will be banned within the European Union for the manufacture of investigational medical products (IMPs) and new MPs beginning on 22 December 2023.
Note that substances containing ≤0.1% (w/w) Triton X-100 surfactant are not considered dangerous and still can be imported to Europe. Because the product typically is not used as an excipient for biologicals or advanced therapies, most such medicines that are produced outside Europe will not need manufacturing changes until their sponsors consider making them in the European Union. Also, already licensed medicines and their excipients are exempt from the rule (for now).
All other manufacturers wishing to produce biological medicines and investigational products in the European Union must seek Triton X-100 replacements and validate their fitness for the respective purpose, most notably in viral reduction applications.
Screening Replacements
The biotechnology industry has reacted to the pending phase-out by searching for suitable substitutes — an activity initiated by larger manufacturers that already use detergent-based inactivation steps. The reported candidates differ in chemistry, application points, and requirements for efficient activity (Table 1). Most of those options are as effective as Triton X-100 surfactant at inactivating X-MuLV (the consensus model for RVLPs) in biopharmaceutical matrices. In some cases, the kinetics of inactivation have been challenged by cold manufacturing or compared with and without solvents (12). Inactivation of other enveloped viruses (e.g., HIV-1, PRV, and BVDV) also has been evaluated in model plasma-product matrices, with results that are equivalent or superior to those seen in biopharmaceutical studies (12, 13).
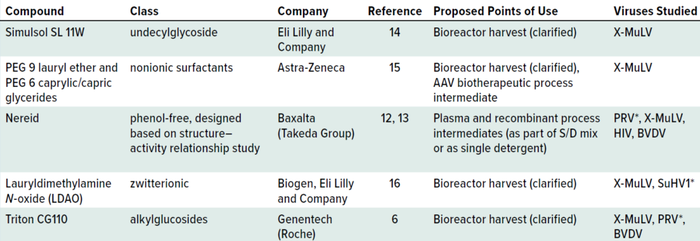
Table 1: Potential Triton X-100 replacements in published scientific literature; PEG = polyethylene glycol, AAV = adenoassociated virus, S/D = solvent/detergent, X-MuLV = xenotrophic murine leukemia virus, PRV = pseudorabies virus, HIV = human immunodeficiency virus, BVDV = bovine viral diarrhea virus, SuHV1 = Suid herpesvirus 1.
* Although PRV and SuHV1 are synonymous, different study authors have preferred one term over the other.
Important Criteria for Candidate Detergents: To be a viable candidate, a Triton X-100 replacement would need to be effective at virus inactivation, preferably against multiple enveloped viruses. Inactivation would need to be robust with respect to the presence of other molecules that can present in HCCF — e.g., lipids, host-cell proteins (HCPs), DNA, and antifoam agents.
Beyond robustness, a number of other criteria should be considered. For example, a detergent needs to be “eco-friendly,” meeting global disposal requirements to replace one that cannot. Sourcing considerations also are critical: The detergent must be economical and, ideally, available from several suppliers. Its production process must be scalable in response to increasing manufacturing demands from biopharmaceutical developers. Raw-material purity standards for pharmaceutical manufacturing would apply, with the attendant need for analytics that can assure purity and other attributes. If a detergent is compendial grade, that would be a bonus.
Replacements should have little or no impact on drug-product quality or bioprocess performance. They must not affect protein drug substances (e.g., by causing precipitation or denaturation). Undesirable or reactive by-products (e.g., peroxides) should be minimized. Further, foaming during mixing should be limited or absent. Detergent removal by downstream processing steps would be expected, with analytics in place to measure levels of the substitute detergent over a wide concentration range. It would need to dissolve efficiently in aqueous solutions. And safe handling in manufacturing is expected, so toxicity must be minimal (including from potential impurities).
Regulatory Considerations: From a regulatory standpoint, validation of unit operations based on alternative detergents for viral clearance would be required — and revalidation of existing production processes if a substitute is introduced. Beyond that, other process validation aspects would need to be addressed at small scale and probably with process-performance qualification (PPQ) batches. Validation studies would need to show that the new detergent affects neither protein nor drug-product stability nor downstream process-fluid intermediates on hold. The revised virus-inactivation unit operation should be demonstrated to provide an acceptable step yield, and tests should demonstrate that detergent does not clog or otherwise affect the efficiency of downstream steps. Detergent clearance would need to be studied at small scale and confirmed in large-scale PPQ runs. The replacement should leave no residues or build-up on column matrices or equipment after routine cleaning.
Preclinical studies would be needed for a revised impurities toxicology assessment. For example, sponsors should recalculate a new impurity safety factor for products they make using a replacement detergent.
All of those activities would need to be reported to and approved by regulatory agencies that had approved the original process. Approval timing disparities around the world inevitably would lead to staged process versions for different countries as the new version gets approved by each regulator.
All those considerations could incentivize a conservative implementation approach at many biopharmaceutical companies, biasing some against changing their existing processes and leaving existing Triton X-100 processes “grandfathered” in place. That would be feasible for existing products according to the EU legislation.
However, for new products, making efforts toward getting ready for this transition is imperative. Getting ready sooner rather than later probably is the best option, not only to alleviate regulatory complexities, but also for the health of our shared aquatic ecosystems.
Acknowledgment
We are grateful to Dr. Michael Craig (Parexel International) for incisive comments on our manuscript.
References
1 Regulation (EC) No. 1907/2006 of the European Parliament and of the Council of 18 December 2006. EUR-Lex 1 May 2022; https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A02006R1907-20220501.
2 Edwards CA, et al. Tri(n-Butyl) Phosphate/Detergent Treatment of Licensed Therapeutic and Experimental Blood Derivatives. Vox Sang. 52(1–2) 1987: 53–59; https://doi.org/10.1111/j.1423-0410.1987.tb02989.x.
3 Griffith M. Ultrapure Plasma Factor VIII Produced By Anti-F VIII c Immunoaffinity Chromatography and Solvent/Detergent Viral Inactivation: Characterization of the Method M Process and Hemofil M Antihemophilic Factor (Human). Ann. Hematol. 63(3) 1991: 131–137; https://doi.org/10.1007/bf01703243.
4 Dichtelmuller H, et al. Robustness of Solvent/Detergent Treatment of Plasma Derivatives: A Data Collection from Plasma Protein Therapeutics Association Member Companies. Transfusion 49(9) 2009: 1931–1943; https://doi.org/10.1111/j.1537-2995.2009.02222.x.
5 ICH Q5A(R1): Quality of Biotechnological Products: Viral Safety Evaluation of Biotechnology Products Derived from Cell Lines of Human or Animal Origin. International Council on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use: Geneva Switzerland, 1997; https://database.ich.org/sites/default/files/Q5A%28R1%29%20Guideline_0.pdf.
6 Durno L, Tounekti O. Viral Inactivation: Low pH and Detergent. PDA J. 69(1) 2015: 163–172; https://doi.org/10.5731/pdajpst.2015.01040.
7 Committee E55 on Manufacture of Pharmaceutical and Biopharmaceutical Products. E3042-16: Standard Practice for Process Step to Inactivate Rodent Retrovirus with Triton X-100 Treatment. American Society for Testing and Materials: West Conshohocken, PA, 2016.
8 REACH Registration Statistics. European Chemicals Agency: Helsinki, Finland, 30 September 2022; https://echa.europa.eu/documents/10162/2741157/registration_statistics_en.pdf.
9 Quirós L, et al. Detection and Evaluation of Endocrine-Disruption Activity in Water Samples from Portuguese Rivers. Environ. Toxicol. Chem. 24(2) 2005: 389–395; https://doi.org/10.1897/04-121r.1.
10 Member State Committee. Support Document for Identification of 4-(1,1,3,3-Tetramethylbutyl)Phenol, Ethoxylated. European Chemicals Agency: Helsinki, Finland, 2012; https://echa.europa.eu/documents/10162/430c2613-588f-8b08-8a72-df4013727ef8.
11 Annex XIV: Authorization List. European Chemicals Agency: Helsinki, Finland, 2022; https://www.echa.europa.eu/authorisation-list.
12 Farcet JB, et al. Development of a Triton X-100 Replacement for Effective Virus Inactivation in Biotechnology Processes. Eng. Reports 1(5) 2019: e12078; https://doi.org/10.1002/eng2.12078.
13 Farcet JB, et al. Synthesis of “Nereid,” a New Phenol-Free Detergent To Replace Triton X-100 in Virus Inactivation. J. Med. Virol. 93(6) 2021: 3880–3889; https://doi.org/10.1002%2Fjmv.26708.
14 Luo W, et al. Identification and Characterization of a Triton X-100 Replacement for Virus Inactivation. Biotechnol. Prog. 36(6) 2020: 3036; https://doi.org/10.1002/btpr.3036.
15 Hunter A, et al. Identification of Compendial Nonionic Detergents for the Replacement of Triton X-100 in Bioprocessing. Biotechnol. Prog. 38(2) 2021:e3235; https://doi.org/10.1002/btpr.3235.
16 Conley L, et al. Evaluation of Eco-Friendly Zwitterionic Detergents for Enveloped Virus Inactivation. Biotechnol. Bioeng. 114(4) 2017: 813–820; https://doi.org/10.1002/bit.26209.
For Further Reading
Scott C. Inactivation of Enveloped Viruses: Seeking Alternatives to a Problematic Surfactant. BioProcess Int. eBook, 9 November 2018; https://bioprocessintl.com/downstream-processing/viral-clearance/inactivation-of-enveloped-viruses-seeking-alternatives-to-octylphenol-ethoxylate-surfactant.
Christiane Niederlaender, PhD, and corresponding author Kurt Brorson, PhD, are technical vice presidents in regulatory consulting services at Parexel International in Newton, MA; 1-240-893-2144; [email protected].
Triton is a registered trademark of Dow Chemical Company. Simulsol is a registered trademark of Surfachem Group Ltd.
You May Also Like