Advances Toward Dry-Powder Formulations for Monoclonal AntibodiesAdvances Toward Dry-Powder Formulations for Monoclonal Antibodies
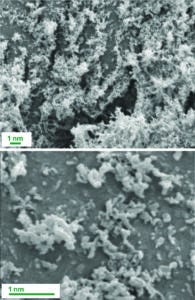
Images of a drug substance formulated using thin-film freezing technology. (IMAGE COURTESY OF THE WILLIAMS LABORATORY AT THE UNIVERSITY OF TEXAS AT AUSTIN AND TFF PHARMACEUTICALS).
Therapies based on monoclonal antibodies (MAbs) and other recombinant proteins usually are formulated as aqueous solutions for subcutaneous injection or intravenous infusion. However, as the biopharmaceutical industry amasses manufacturing knowledge and experience with such products, interest is surging for alternative formulation methods. Aerosolizable dry powders for inhalable administration represent a particularly promising option, with potential advantages not only for drug delivery and dosing, but also for patient comfort and compliance with treatment regimens.
Previous research into dry-powder biopharmaceutical formulation has focused largely on nasally delivered vaccines and pulmonary-directed antivirals and antibiotics. But such drugs remain underrepresented among marketed products (1–4). Inhalable MAbs have even further to go to reach patients, although the COVID-19 pandemic accelerated efforts to formulate MAb-based therapies for direct delivery to lung tissue and nasal passageways.
At the height of the recent public-health emergency, I spoke with Robert O. Williams III — who is division head and professor of molecular pharmaceutics and drug delivery at the University of Texas at Austin (UT Austin), as well as a scientific advisor to TFF Pharmaceuticals — about advances in dry-powder formulations of vaccines (5). I caught up with him again early in 2023 to learn about his laboratory’s work with MAbs and other proteins. Williams explained pharmacokinetic and stability-related advantages associated with dry-powder formulations and described available technologies for producing such formulations. He also highlighted a great need for an expanded “toolbox” of solutions that would help drug makers to select the formulation method that makes the most sense for a given therapeutic protein.
Improving Protein-Product Stability
Why are MAbs almost exclusively formulated as liquid suspensions? Is that the easiest approach to take? It’s definitely not an easy formulation method, but large proteins such as MAbs cannot penetrate gastrointestinal lining and skin tissue, nor can they pass easily through mucosal layers. Thus, therapeutic proteins usually are delivered by infusion or injection — and formulated either as liquids or freeze-dried powders for reconstitution — to reduce barriers during administration.
But as with small-molecule drugs, therapeutic proteins tend to be chemically unstable when formulated as liquid solutions. Drug substances are susceptible to chemical degradation, which can lead to reduction of biological activity. Proteins also can degrade physically, manifesting as aggregates and high–molecular-weight (HMW) species (which sometimes are called subvisible aggregates). Such degradation products can raise toxicity and immunogenicity concerns upon injection.
What advantages could dry-powder formulations provide over liquid solutions for protein-based drug products? Formulation as a dry powder helps to alleviate the chemical and physical degradation that occurs when proteins are formulated into a liquid (typically an aqueous solution). Because dry powders show increased chemical and physical stability, they enable administration of therapeutic proteins to the lungs using a dry-powder inhaler. Powder-formulated drug products also can be delivered to nasal passageways. Our laboratory has worked on that approach, and we recently explored nasal applications of therapeutic proteins (6).
Liquid formulations can be adapted for inhaled delivery by nebulization, but that process tends to generate high shear, which for many kinds of proteins results in degradation. When applying dry-powder inhalers, our team has observed no such degradation across many types of proteins.
Another advantage is that dry powder enables direct drug delivery. If you can deliver a therapeutic to the disease source — e.g., to lung or nasal tissue in which a bacterial or viral infection has developed — then you can apply a much lower dose than would be needed for systemic administration. Pulmonary or nasal delivery might require 1/5 or even 1/10 of a standard dose to be effective, so drug makers could save considerable amounts of drug product by leveraging inhalable formulations. Such presentations would be far less invasive than injectable products are. Injections and infusions require administration by highly trained healthcare professionals who have access to the requisite equipment (e.g., needles and tubing for intravenous infusion). Thus, in our laboratory, we try to leverage dry-powder formulations when doing so makes sense for the disease and therapeutic agent at hand.
I know about a few companies that are creating dry-powder biopharmaceuticals, but such products usually have vaccine applications. Why has it been difficult to formulate therapeutic proteins into powders? The technology for dry-powder generation has been insufficient — although it has evolved rapidly during the COVID-19 pandemic, spurred on by the development of many different therapeutics and vaccines. Our team works primarily with thin-film freezing (TFF) technology, which is under commercial development with TFF Pharmaceuticals. Besides that approach, drug manufacturers can perform spray drying (SD) or spray freeze drying (SFD). The former method atomizes a liquid solution into micron-sized droplets and then applies heat to dry them. SFD also atomizes solutions but freezes the droplets; then, the frozen liquid is sublimated off.
Some proteins are amenable to SD and SFD, but in my experience, many protein products will not work in such processes. When our team began developing TFF technology, we recognized that droplets created by SD and SFD have relatively high surface-area-to-volume ratios, giving proteins significant access to air–liquid interfaces. Proteins tend to be surface-active, so they accumulate at such interfaces, where hydrophobic interactions can cause them to denature. We designed the TFF method to eliminate that problem. The technology generates millimeter-sized droplets that, because of their large size, minimize protein exposure to air–liquid interfaces. Thus, we have observed far less denaturation among TFF-formulated proteins than we have with SD- and SFD-based formulations.
For instance, our team recently compared TFF- and SFD-formulated droplets containing the surface-active protein lactoferrin. We showed conclusively that lactoferrin accumulates at the air–liquid interface in micron-sized SFD-generated droplets but not in particles created by our platform (7). Because of that factor, TFF-formulated proteins receive a significant advantage in preserving chemical activity and stability.
Emerging Methods for Generating Dry-Powder MAb Formulations
How does TFF technology work? Our process begins with a liquid-formulated protein product — typically a buffered aqueous solution. To prevent degradation, we limit how long a product remains in solution. Our system forms the liquid into millimeter-sized droplets. Upon exposure to an intermediate freezing rate on a frozen substrate, those droplets form frozen thin films. Then, we will sublimate the frozen solvent, leaving a powder with a high surface area. We call that powder a “brittle matrix” because its constitutive particles are small but interconnected. Because of that structure, the particles have excellent aerosol properties for inhaled delivery. When emitted from a dry-powder inhaler, the powder readily and rapidly breaks up into aerodynamically favorable aggregates. Those aggregates are large and light enough to reach tissues deep in the lung regardless of a patient’s ability to take a deep breath from the device. The powder also reconstitutes quickly (10–20 seconds).
Our researchers have made several discoveries when comparing TFF-generated formulations with lyophilized products. For instance, the TFF method’s drying rate is about 2× faster than that of lyophilization, and even before sublimation, frozen thin films have a higher surface area that, compared with lyophilization, enables homogeneous drying (8).
Equally interesting is that TFF technology could address concerns about microbubbles in protein-drug formulations. Literature about the problem is abundant, and the field of drug-product formulation has yet to present an effective solution (9). Bubbles often are created during slow freezing processes because air is drawn out of a solution at the liquid–ice interface. Some of those bubbles are quite large, and again, proteins do not like interfaces. Using TFF methods, we can minimize microbubble formation, increasing freezing-process stability. We are beginning to leverage such advantages to create better, more stable formulations.
How does your team evaluate absorption, distribution, metabolism, and excretion (ADME) properties? For instance, how do you ensure that a TFF process yields correctly sized particles? We perform considerable laboratory testing. First, we ensure that a drug will be chemically and physically stable upon inhalation. Then, we determine an optimal aerodynamic particle size, such that a powder formulation can be inhaled into the deep lungs or delivered to nasal passageways effectively.
We take great care when conducting such tests. But that approach requires rigorous trial and error, which consumes time and money — and involves many mistakes. About five years ago, a graduate student from our team interned with Merck to learn about applications of artificial intelligence (AI). That is when I was introduced to machine learning (ML) and its potential for our laboratory’s work. Soon after, our team partnered with researchers at UT Austin’s computer science department. Subsequently, we’ve expanded the collaboration outside of UT Austin. In essence, we are trying to leverage “big data” for study of formulation parameters. For example, we recently worked with ML to predict aerodynamic performance (10). We wanted to know how efficiently TFF-generated powder could move from an inhaler into the deep lung, for both nonhuman animal models and humans.
Our laboratory has worked with multiple drugs over many years, so we have considerable data sets. The student I mentioned gathered those data, and we analyzed them to develop different representative models. We determined — as suspected — that TFF-generated powders perform predictably. Then, we established parameters to measure and created an effective model. Doing so has reduced our trial-and-error time in the laboratory considerably.
The Future of Drug-Product Formulation
Could TFF technology work for other biopharmaceutical modalities? We are working with multiple modalities, including different MAbs, cytokines, and growth factors, as well as messenger and small interfering RNA (mRNA and siRNA) (11). We’re even applying the technology to bacteria and other live microbes (12). Since we introduced the technology to BPI’s readers, we’ve tried to expand its applications significantly.
What aspects of TFF technology require further study? TFF Pharmaceuticals continues to work on scaling up manufacturing processes based on what we do in our laboratory units. The company has formulated several drug products that are undergoing phase 1–2 clinical trials, including inhalable TFF-generated powders of voriconazole and tacrolimus (13).
In the laboratory, we have demonstrated that water is removed much more quickly from frozen thin films than from shelf-frozen bulk solids produced by conventional freezing and lyophilization (8). That is because frozen thin films are not as thick and have a much lower surface-area-to-volume ratio, both of which facilitate sublimation and drying processes. Those findings surprised us, so now we are leveraging such capabilities to speed up our formulation processes.
You might ask why process acceleration would be a big deal. Of course, it represents time and money, but also sublimation can diminish protein stability. Improvements to process efficiency could help to preserve the stability of proteins that have difficulty undergoing conventional sublimation.
As I mentioned, we have demonstrated that SFD processes can introduce small air bubbles near liquid–ice interfaces. Through the ingenuity of our postdoctoral researchers, we have visualized the denaturation of surface-active proteins that diffuse and “stick” to those hydrophobic interfaces. Now that we understand the problem more completely, we have developed a method that prevents formation of hydrophobic air bubbles. As a result, some of our model surface-active proteins are showing improved stability characteristics.
We continue to expand the types of protein-based biologics to which TFF methods can be applied. Each biologic presents unique formulation challenges. Our group’s goals are to dive into the properties of given proteins, test several options — and make many mistakes — quickly, and then develop strategies that can be applied to different types of biologics. We want our work to be more than trial and error; we want to devise general formulation principles based on results from our experiments.
For biological products, few technologies are available for converting liquids into powders. TFF is one such technology, and we believe that it raises several advantages for protein biologics. But researchers need to fill the toolbox with different methods for particle engineering and conversion of liquids into dry powders. No formulation process will work for all biologics. We must have choices available to address the properties of different proteins.
References
1 Scherließ R. Nasal Formulations for Drug Administration and Characterization of Nasal Preparations in Drug Delivery. Ther. Dev. 11(3) 12 February 2020; https://doi.org/10.4155/tde-2019-0086.
2 Romeo VD, et al. Effects of Physicochemical Properties and Other Factors on Systemic Nasal Drug Delivery. Adv. Drug Deliv. Rev. 29, 1998: 89–116; https://doi.org/10.1016/s0169-409x(97)00063-x.
3 Cipolla DC, Gonda I. Formulation Technology To Repurpose Drugs for Inhalation Delivery. Drug Discov. Today 8(3–4) 2011: 123–130; https://doi.org/10.1016/j.ddstr.2011.07.001.
4 Telko MJ, Hickey JA. Dry Powder Inhaler Formulation. Resp. Care 50(9) 2005: 1209–1227; http://rc.rcjournal.com/content/50/9/1209.short.
5 Scott C, Gazaille B. Formulation, Fill–Finish: Biopharmaceutical Drug-Product Trends and Technologies. BioProcess Int. eBook, January 2021; https://bioprocessintl.com/manufacturing/fill-finish/ebook-formulation-fill-finish-biopharmaceutical-drug-product-manufacturing-trends-and-technologies.
6 Hufnagel S, et al. The Development of Thin-Film Freezing and Its Application To Improve Delivery of Biologics as Dry Powder Aerosols. KONA Powder Particle J. 39, 2022: 176–192; https://doi.org/10.14356/kona.2022010.
7 Dao HM, et al. Aggregation of Lactoferrin Caused by Droplet Atomization Process via a Two-Fluid Nozzle: The Detrimental Effect of Air–Water Interfaces. Molec. Pharm. 19(7) 2022: 2662–2675; https://doi.org/10.1021/acs.molpharmaceut.2c00358.
8 Wang J-L, et al. Accelerated Mass Transfer from Frozen Thin Films During Thin-Film Freeze-Drying. bioRxiv, 17 April 2022; https://doi.org/10.1101/2022.04.16.488553.
9 Dao HM, et al. Entrapment of Air Microbubbles By Ice Crystals During Freezing Exacerbates Freeze-Induced Denaturation of Proteins. Int. J. Pharm. 628, 2022: 122306; https://doi.org/10.1016/j.ijpharm.2022.122306.
10 Jiang J, et al. The Applications of Machine Learning (ML) in Designing Dry Powder for Inhalation By Using Thin-Film-Freezing Technology. Int. J. Pharm. 626, 2022: 122179; https://doi.org/10.1016/j.ijpharm.2022.122179.
11 Xu H, et al. Aerosolizable Plasmid DNA Dry Powders Engineered by Thin-Film Freezing. Pharm. Res. 26 January 2023; https://doi.org/10.1007/s11095-023-03473-5.
12 Wang J-L, et al. Accelerated Water Removal from Frozen Thin Films Containing Bacteria. Int. J. Pharm. 630, 2023: 122408; https://doi.org/10.1016/j.ijpharm.2022.122408.
13 Programs. TFF Pharmaceuticals: Fort Worth, TX, 2023; https://tffpharma.com/programs.
Brian Gazaille, PhD, is managing editor of BioProcess International; [email protected]. Robert O. Williams III is division head and professor of molecular pharmaceutics and drug delivery at the University of Texas (Austin), scientific advisor to TFF Pharmaceuticals, and editor in chief of the American Association of Pharmaceutical Sciences (AAPS) journal PharmSciTech.
You May Also Like






