Artist conception of T cells attacking a cancer cell (www.istockphoto.com)
Just about anyone in the biopharmaceutical industry will tell you that cost is now the primary concern in cell and gene therapy development. It hasn’t even been a decade since “manufacturability” was the main issue at hand — and cost has risen organically from related discussions. Regenerative medicine evolved from medical research rather than from drug-development companies, and technologies that worked in clinical settings haven’t translated directly to manufacturing facilities. Cost is often the problem. Early product successes (that ultimately weren’t so much) made the importance of cost control glaringly clear. And whereas the classical drug field increasingly is called out for massive mark-ups and huge advertising budgets, the price of regenerative medicines truly is linked directly to the cost of making them.
Some experts rightly have pointed out that it’s not fair to compare the cost of a one-time cure (e.g., for cancer) with that of a treatment that goes on indefinitely (e.g., for high blood pressure). Taken in total, the cost of a lifetime course of diabetes treatment — not just in sheer dollars but also in commitment, time, effort, and quality of life — would be quite high. The goal of the cell and gene therapy industry should be to offer a better alternative.
This is the dilemma facing developers of chimeric-antigen receptor (CAR) T-cell therapies: not only to make that case, but also to last long enough to make it. Many such companies are dependent on angel investors, venture capital, or partnerships with larger entities to pay their bills as they work toward that goal. If the money runs out, then no matter how promising the technology, it will never have a chance to help anyone. On the other hand, investors want to get involved with organizations that have real promise. They want to know not only that an idea is a good one, but also that the people working on it know what they’re doing and how to realize that potential.
Chemistry, Manufacturing, and Controls (CMC): Cost consciousness can be dangerous, however. Development of injectible products — often for patients who are not at their strongest — is rightfully subjected to close regulatory scrutiny. The making of a CAR T-cell therapy is a highly complex endeavor requiring both a gene-therapy manufacturing process (often including viral vectors) and one for collecting T cells, genetically modifying and expanding them, and preparing them for patients. These multistep technologies and logistics are rife with risk. Sometimes the people working on these products can be so focused on results that they don’t think enough about how those are achieved. And when it comes time to explain both, companies can fall short of what regulators want to see and current good manufacturing practice (CGMP) requires.
In early December 2019, the US Food and Drug Administration (FDA) rejected a market application from Enzyvant for a tissue therapy (arguably a less complex regenerative medicine than CAR T cells) to treat a rare immunodeficiency condition primarily because of manufacturing concerns. As RoosterBio founder and chief technology officer Jon Rowley commented on LinkedIn, “I can’t tell you how many times I have heard over the years that if you get the clinical data, the FDA will be lenient on manufacturing matters. I think we can now put this myth to rest.” His post drew much interest and a number of highly salient points from other experts in the regenerative medicine field (1):
“. . . a classic example of how thorough due diligence is required for drug manufacturing irrespective of its phase.” — a project and business manager
“The product is the process; the process is the product.” — a head of process development
“CMC matters. If you do not control your process, if it is not consistent or the fail rate is too high, then you will have a problem . . . Having a postcommercialization improvement plan is fine, but only if the process is under control already.” — an industry consultant
“Poorly executed CMC is and should be a showstopper. . . too often underrated and underfunded.” — an industry consultant
“Wondering if working with a more reputable CMO could have avoided this.” — a head of commercial development and active pharmaceutical ingredient (API) manufacturing
“It’s also perhaps a sign of the increasing agency knowledge and stringency that goes with that. I have heard many times that you can ‘catch up’ with CMC, and it’s a very dangerous way of thinking.” — a head of regulatory affairs
“While adherence to quality and manufacturing standards/requirements currently may be challenging for regen-med therapies, there is no ‘pay later/will comply later’ plan. The industry went through the same growing pains a few decades ago with biologics until we got a better understanding of how to apply small-molecule requirements to recombinants and monoclonals.” — an industry consultant
“There always will be fundamentals to fulfill for any medicine CMC with respect to process control, safety aspects, and adherence to CGMPs and legal requirements. Flexibility exists, but it needs to be understood through dialogue with regulators rather than assumed.” — a vice president of regulatory affairs and quality assurance
“You can overdo it,” Rowley continued in reply to that last comment, “and those who put too much into CMC can burn through too much money and time. That impedes competitiveness — or they run out of money. However, it is critical to put sufficient effort in so that you have responsibly understood your product and process. It is a balance, for sure.”
BWB Speakers Highlight the Latest Progress and Concerns
Informa Connect’s 2019 Biotech Week Boston (BWB) festival included a full conference program titled, “Cell and Gene Therapy Bioprocessing and Commercialization.” A number of speakers there discussed different aspects of CAR T-cell therapy development. The following examples show that although industry is putting much effort into advancing these technologies, academic and medical research organizations still are heavily involved — and many of the latter have come to realize that manufacturing concerns are just as important as “pure research” in getting these therapies from the realm of possibility into the reality of treating patients. Note that some cancer treatment centers now are building their own manufacturing facilities (see the Orgenesis box for more on that).
Genetic Enhancements: In “Enhancing Tumor-Directed T Cells with an Interleukin 7 Signal Modulator,” Bilal Omer (physician at Texas Children’s Cancer Center) touted the ~80% cancer remission rate for approved CAR-T cells in treatment-refractory hematological diseases. He also lamented their limited efficacy in treating solid tumors. Shown in preclinical studies to enhance cell expansion and serial cytotoxicity against different types of tumors, the interleukin-7 signal modulation approach he called “C7R T-cell therapy” is moving into clinical trials now. It adds an interleukin protein to the CAR genetic modification of T-cells to help them bind to cytokine receptors on solid-tumor cells.
Culturing Cells: In “Establishing the Scalable Manufacture of Primary Human T Cells in an Automated, Stirred-Tank Bioreactor,” Elena Costariol (biochemical engineering researcher at University College London, UCL) highlighted her laboratory’s collaborative work with GlaxoSmithKline, MedImmune, and Pall Biotech as well as spinout companies Puridify, Synthace, and kompAs/Gowerlabs. She also described a future “targeted-healthcare manufacturing hub” that will be paid for by a £10 million grant over the period of 2017–2024. With the biopharmaceutical industry moving from “one-size-fits-all” medicines toward personalized therapies, UCL professor Nigel Titchener-Hooker and his team are working on manufacturability.
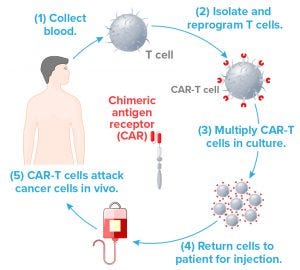
Figure 1: In this familiar simplified version of the CAR T-cell therapy process, each step depends on a number of logistic and manufacturing aspects for success. For example, Step 2 requires gene vectors that typically can be produced in large quanitites to serve many patients, even though the ultimate CAR-T product is autologous. Step 3 requires specialized media and hardware. Steps 1 and 4 require validated leukapheresis procedures and documented tracking with no room for error.
Costariol’s talk focused on the culture of primary human T cells in an ambr 250 microbioreactor from Sartorius Stedim Biotech — a technology for Step 3 in Figure 1. She pointed to the high incidence of manufacturing errors, product variation, and contamination associated with open manufacturing and manual handling of laboratory equipment such as culture flasks. One step toward lowering costs would be to eliminate such problems through automated processes.
“Advances in automated technology and process understanding can facilitate scale-up and enable successful translation,” she said. But culturing primary cells (collected in vivo) is never as easy as culturing cell lines adapted to in vitro conditions. Luckily, T cells circulate in our bloodstreams and thus do not need to attach to a support to grow and reproduce. Costariol’s data showed that cells isolated from healthy donors could be grown in stirred-tank bioreactors. Expansion was significantly better in an ambr 250 vessel agitated at 200 rpm than in a static control flask. Using flow cytometry to analyze subpopulations showed that the phenotypes were comparable. Stirring did not affect expression of the CAR receptor or compromise the cells’ killing ability.
In “Pathogen-Reduced Human Platelet Lysate As a Serum Replacement for Ex Vivo Expansion of CAR-T Cells,” Norihiro Watanabe (instructor in the Center for Cell and Gene Therapy at Baylor College of Medicine) focused on the media support for cells in culture.
T cells must be stimulated to multiply, usually by adding cytokine proteins to their suspension before transducing with a genetic vector. In first-generation regenerative medicine processes, cells were cultured in a patient’s own serum. Now groups like Watanabe’s are investigating other options. He showed enhanced proliferation and prolonged survival in vivo as benefits of using human platelet lysate with CAR-T cells, for example, with increased antitumor potency as a result. And his group plans to test the medium with other immune cells.
Analytical Approaches: In “Leveraging High-Dimensional ‘-Omics’ Technologies for Comprehensive Profiling of CAR-T Cells to Resolve Drug-Product Complexity,” Eric Alonzo (senior scientist in cell analytics at bluebird bio) emphasized the importance of CMC in regulatory applications for cell and gene therapy products. In particular, he pointed to the chemistry aspects, quoting FDA commissioner Scott Gotlieb, who said at a June 2018 meeting, “It’s the product questions that are more complex and uncertain.”
Alonzo said that regenerative medicine companies need to ask two specific questions: “What’s in the bag?” and “How well did the process work?” The former refers to which cells make up a product lot, and the latter to methods of characterization. He showed how mass cytometry could provide more detailed phenotyping than classical flow cytometry, and how single-cell RNA sequencing (scRNA-seq) would be better than “bulk RNA sequencing.” When each cell type has a distinct expression profile, heterogeneities and subpopulations can be revealed to show the real variability in large pools of cells.
Future work at bluebird will use GMP samples from products in clinical trials, integrating even more “-omics” technologies for deep characterization and biomarker discovery. Alonzo said assay development continues for increasing both manufacturing and product knowledge. His team wants to correlate clinical data and assays with CMC characterization data to identify biomarkers that define signatures for efficacy and relapse.
In “Addressing the Challenges of Cell and Gene Therapy Commercialization,” Øystein Åmellem (head of cell therapy R&D at Thermo Fisher Scientific) explained that current cell and gene manufacturing paradigms will need substantial innovation to meet global requirements and demand. Carefully designed comparability studies will be necessary to ensure smooth transitions from older to newer technologies. He emphasized that product developers will need to know their products and processes intimately, and that requires well-understood analytical methods and controls. “Consider additional analytical methods for deeper process and product characterization,” he said. Risk assessment will help manage inherent variabilities in starting materials through testing and use of relevant statistical methods. Open communication with regulators is key.
Overall Operations: In “Development of CAR-T Cell Manufacturing,” Xiuyan Wang (director of the cell therapy and cell engineering facility at Memorial Sloan Kettering Cancer Center) illustrated the real complexity of assembling CARs for T-cell therapy. From target selection through genetic engineering, tumor immunology, and synthetic receptor design to cell manufacturing, all operations require regulatory compliance. She listed many instruments and technologies involved in cell collection and selection (e.g., cell processing systems and magnetic-bead separation systems); activation and gene transfer (e.g., viral vectors and electroporation systems); and expansion, formulation, and cryopreservation (e.g., cell culture vessels and controlled-rate freezers). Then she showed how most of those steps can be automated, and how, with the right equipment, an entire process can be complete in about a week.
“A modular CAR-T manufacturing platform has successfully supported our phase 1–2 clinical trials,” she said, naming the closed and automated CliniMACS Prodigy system from Miltenyi Biotec as the replacement for that platform. With a capability to manufacture a number of cell types in a single process set-up, it provided expansion, viability, and transduction efficiency comparable to those of the established platform. “CAR-T cells generated from the Prodigy platform showed comparable or superior antitumor activity in vivo,” she reported.
Conversations on Cost and Concerns
In November 2019, Claudette Hodge of BPI’s parent company Informa spoke with three industry experts who later spoke at Informa Connect’s “Cell Therapy Manufacturing and Gene Therapy Congress” in Amsterdam during the first week of December (https://informaconnect.com/celltherapy). “Having raised the bar in immunotherapy,” she wrote, “CAR T-cell therapy is now one of the most promising and sought-after cancer treatments” (2). But the exclusively autologous format is burdened by high costs and challenges in scaling up to meet demand. Companies such as Gilead Sciences and Kite Pharma are racing to develop allogeneic or “off-the-shelf” variations on the theme, but safety and efficacy concerns also remain. Hodge asked Xiuyan Wang (assistant director at Memorial Sloan Kettering Cancer Center), Benoit Bossuge (strategic project and account manager at Novartis Technical Operations), and Philippe Parone (director of industrialization at Celyad) to comment on what can be done to make these therapies more affordable in the meantime — and how far away a universally accessible CAR-T therapy might be.
Hodge: What are the most effective ways to reduce CoG for CAR-T therapies?
Wang: I think automation, better CAR design (hence lower required cell doses), decreased paperwork (hence fewer quality assurance staff), and simpler manufacturing flow/platforms all will reduce CoG.
Bossuge: Closed systems and standardized platforms along with automation are the future. A great deal of innovation is taking place to reduce costs and complexity and to improve processes in manufacturing of cell and gene therapies. Improving manufacturing efficiency through automation is one of the big challenges that will need to be addressed to reduce CoG. The rise of smart technologies including development of smart packaging as a supply-chain solution (e.g., using blockchain technology) also will play a key role.
Parone: Manufacturing of CAR T-cell therapies is a complex process with a high CoG, which is likely to be a challenge for the long-term sustainability of commercialized CAR-T products. Nevertheless, several approaches can be used to tackle CoG, including labor and raw material costs. One such approach is automation of manufacturing processes and analytical methods. Automation reduces not only labor costs, but also manufacturing deviations and batch failures, both of which can drive CoG. Process automation also enables scale-out, which maximizes use of raw materials and facilities.
Of course, the most efficient way to reduce CoG for CAR-T therapies would be to transition to allogeneic products, providing that they have comparable efficacy and safety. Manufacturing of allogeneic products benefits from multiple factors that contribute to lowering CoG, including a simplified supply chain, economies of scale on raw materials, and reduced quality control (QC) costs for release. One approach to optimize CoG for allogeneic products is to increase batch size without substantially changing labor and material costs. That will decrease patient-dose costs proportionally to the increase in batch size. Manufacturing yield can be optimized using in-line monitoring sensors to adjust culture conditions in real time for maximal expansion. Enhancing gas exchange during culture is another way to maximize growth.
Those are only a few approaches that can be tested to tackle CoG for CAR T-cell therapies. Each comes with a development cost, and it is a balancing act to find the right timing to test and implement cost-saving measures.
Hodge: How do you see the accessibility for CAR-T therapies changing in 2020?
Wang: I think more CAR T-cell products will be approved, which will increase accessibility of such therapies. However, if the cost of CAR-T therapies remains as high as it is today, it still will be a major barrier to broader application.
Parone: High price tags and limited reimbursement for CAR T-cell therapies are hurdles to patient accessibility. Nevertheless, there is a continual drive to increase patient access to these life-saving therapies. Certain events that took place in 2019 should expand that access in 2020. For example, August saw the Centers for Medicare and Medicaid Services in the United States raise reimbursements for CAR T-cell therapies, thus lowering the financial threshold for access. The same effect is likely to result from a decision by Novartis to provide Kymriah (tisagenlecleucel) at a discounted price in Japan following its approval in May. Facilitating reimbursement also could come from innovative payment models such as the payment-for-outcomes concept recently proposed by Gilead Sciences, which ties a therapy’s clinical success to its payment.
Accessibility of CAR T-cell therapy to patients also is limited by the complex nature of such treatments and associated side effects. Thus, CAR T cells are available only at certified centers, and the patient-referral system is particularly complex. To offset that complexity and favor patient access, Gilead Sciences is encouraging identification of Yescarta (axicabtagene ciloleucel)–eligible patients by informing physicians and helping them with patient referrals. From a safety perspective, the unified grading system for toxicities associated with CAR-T therapies released by the American Society for Transplantation and Cellular Therapy early in 2019 should streamline toxicity treatment across sites. New approaches currently being tested for treatment of toxicities related to CAR T cells (e.g., early intervention using a siltuximab–anakinra combination) should contribute to enhancing the safety of CAR-T treatments, thus increasing their accessibility to patients.
So there are multiple barriers to accessing CAR-T therapies, but recent decisions with regard to cost of treatment, reimbursement, and management of side effects are likely to favor patient accessibility in 2020.
Engineering Solutions: Jenna Balestrini is head of precision medicine and cell bioprocessing at Draper, a not-for-profit engineering innovation company focused primarily on space and national security issues. The company is developing an end-to-end cell therapy bioprocessing system for clinical use. It uses high-precision microfluidics to perform acoustophoretic cell separation, gene delivery by transduction or electrotransfection, and in-line washing. The module continuously and rapidly removes interfering cell contaminants without compromising cell health. Gentle acoustophoresis improves cell yields and accelerates delivery to downstream process steps.
In November 2019, I spoke with Balestrini about CAR-T cost concerns.
Scott: Why is the cost of cell-therapy manufacturing so high?
Balestrini: One reason CAR-T treatments are so expensive is that the industry does not have a “fit-for-purpose” automated manufacturing process that can accommodate the wide variety of workflows needed to produce cell therapies. As a result, there is overreliance on instrumentation and processes that have been borrowed from other modalities such as blood banking and stem-cell transplantation. That results in additional steps, longer production times, and/or low yields.
Additionally, because cell therapy manufacturing processes primarily came out of academic institutions, there is significant “touch labor” involved. In academia, graduate-student labor is inexpensive; trained professionals in industry come at a much higher cost. A high degree of touch labor also contributes to a breakdown in therapy development, increased contamination, and hinders scalability. The field of tissue engineering suffered broadly for the same reasons. And the workflows developed by academics rely on significant know-how and experience that can be very challenging to automate. In a field that has yet to determine its ideal manufacturing conditions, a singular “end-to-end” system would need tremendous flexibility in terms of process and scale.
Scott: How do analytical testing requirements compare with those for other biologics?
Balestrini: Testing of these products is much like that of any other biotherapeutic. The difference is that, with current CAR-T therapies, each patient’s cells equate to a single “batch” of product. Thus, the costs associated with analytical testing and release are borne by the patient for whom a given therapy is being developed.
Scott: Are there special considerations — regulatory, or otherwise — for CAR T-cell therapies separate from other regenerative medicines?
Balestrini: Regenerative medicine replaces, augments, repairs, or replaces failing parts of the body to restore normal function. This can include tissue-engineered applications, biomaterials, gene therapies, and cell therapies. Draper has developed cell-bioprocessing technology to produce cell therapies and enable tissue-engineered applications such as artificial organs, making it possible to leverage a patient’s own material to treat disease.
One thing that separates patients receiving CAR-T cell therapies from those receiving other regenerative-medicine options is that the CAR-T therapies are available only to individuals who have undergone rounds of chemotherapy and radiation, making CAR-T a second- or third-line therapy. Evidence from David Barrett’s group at the Children’s Hospital of Philadelphia (among others) shows that CAR-modified immune cells from patients who have undergone radiation and chemotherapy perform significantly worse as cancer-fighting agents than those from patients who have never received chemotherapy or radiation. As CAR-T becomes a first-line therapy, you will see two things arise: a broader range of applications and improved efficacies for these therapies, and better results from cell immunotherapies because of better starting materials, leading to higher probability of success.
Scott: What are the most effective ways to reduce CoG for CAR-T therapies?
Balestrini: Current instrumentation and methods for modifying genes for cellular therapies are expensive, time-consuming, difficult to scale, and limited in their ability to deliver genetic material effectively. It’s a bioprocessing bottleneck that threatens to hinder both product development and patient access. Reducing CoG requires reengineering an entire biomanufacturing process, integrating a complicated, multistep process into a closed, modular, benchtop system to enable safe and effective biomanufacturing near patients.
Another main factor driving the prohibitive expense of CAR T-cell therapies is the cost of viral vectors, which serve as delivery vehicles of genomic materials into specific cells. These can account for up to 75% of a treatment’s manufacturing cost and are themselves difficult and costly to manufacture. Draper has developed a microfluidic transduction device (MTD) to enable companies to manufacture T-cell therapies with half the viral vectors required by many other approaches.
Such improvements to processes and technologies can help pharmaceutical companies solve the engineering challenges of production to achieve the cost reductions, accelerated results, reduced risk, and increased safety that they need to make cell therapies practical.
Scott: Are most CAR-T developers outsourcing their manufacturing or keeping it in-house?
Balestrini: I think the rapid expansion of the cell and gene therapy pipeline is creating constraints on access to contract capacity. Demand stems from both the expansion of drug-development pipelines and a trend in small startups discovering a growing portion of new product candidates. I think that a growing segment of developers want to own their processes. Whether they can afford to invest in all the infrastructure, manufacturing equipment, space, and personnel needed to do that is up for discussion. It is a huge initial investment, and early stage companies (or those located where real estate is prohibitively expensive such as in Cambridge, MA) don’t always have that luxury.
Scott: Which patients will benefit most from Medicare’s decision to pay for CAR T-cell therapies?
Balestrini: The decision to support Medicare patients for CAR T-cell therapies will expand patient access in treatment of leukemia and lymphoma. The Centers for Medicare and Medicaid Services (CMS) now cover autologous treatments for cancer with T-cells expressing at least one CAR when they are administered at healthcare facilities enrolled in the FDA risk evaluation and mitigation strategies (REMS) program.
They must be used for medically accepted indications or for other uses only when products have been FDA-approved and the use is supported in one or more of the CMS-approved compendia. Current indications for CAR-T therapies are certain types of lymphomas and B-cell leukemias. Given that Medicare is available primarily to patients over 65 years of age, they will be the primary beneficiaries.
Of the cancer indications currently approved, most patients fall within a specific age group. Although lymphomas can occur at any age, the American Cancer Society said in 2018 that more than half of lymphoma patients are 65 or older at the time of their diagnoses. Chronic lymphoblastic leukemia (CLL) also affects more Medicare-eligible patients, given that the average age of onset is 71 years. It is a slower progressing type of cancer, so treatment in that patient population must be weighed against other comorbidities that could be present. Conversely, another approved indication is acute lymphoblastic leukemia (ALL), which affects a much younger population. The median age for an ALL diagnosis is 15 years according to the National Cancer Institute, and only ~11% of patients are diagnosed at 65 years or older.
Scott: How do you see the accessibility for CAR-T therapies changing in 2020?
Balestrini: We need to think more critically about ways to broaden the abilities, precision, and persistence of these adoptive cell therapies to treat not just approved hematologic blood cancers, but also those patients who don’t have enough time or starting materials to make high-quality immunotherapies. Further, we need to come up with a scalable system that is much more specific and effective in terms of its capability of treating other kinds of cancers.
So to expand patient access beyond what has been currently approved the starting materials need to be better, and the biology needs to be tuned toward treating additional cancers. To treat solid tumors, for example, the cell-based therapy can’t introduce rejection via graft-versus-host disease (or in the case of allogeneic processing, host-versus-graft disease). In metastatic cancer, you need something that will work on both a primary tumor and its metastases. Those could have very different genetic profiles and could require different cell-based or combinatory solutions. Such an approach could be very expensive, again highlighting the need for precision manufacturing that enables scalability of these lifesaving drugs.
Expanding patient access to immunotherapies by shrinking processing time to days (from weeks) and costs to a fraction of current amounts would be key benefits of using an end-to-end bioprocessing system. Significant progress may not happen in 2020, but the promise of improving patient access to CAR-T therapies is real. In the near-term, more cell therapies will be introduced and made available to treat more patient populations, not just the current ones.
Scott: How will immunotherapy look in the future?
Balestrini: I predict immunotherapies such as CAR-T will reach much broader patient populations. Removing the bioprocessing bottleneck already is showing promise for improving procurement and production for tumor infiltrating lymphocytes (TILs), T-cell receptors (TCRs), CARs, and neoantigens. Significant advancements are happening with the use of genetic assessment to determine personalized cell therapy solutions for specific patients.
Other Technologies: Please see the “Interview” boxes for further discussions with other technology providers: Orgenesis, Polyplus-transfection, and TrakCell.
But Wait, There’s More!
The authors in this featured report address different aspects of the “CAR-T conundrum.” First, representatives of the International Society for Cell and Gene Therapy provide an overview of cell-banking concerns from a combined regulatory, ethical, and business perspective. Then consultant John Godshalk reviews the CMC aspects of cell and gene therapy regulation and guidance in the United States and Europe. Finally, BioProcess Insider contributors provide a wrap-up of 2019 news in the CAR-T world — including a brand new interview with Zelluna’s chief executive officer, Miguel Forte, on T-cell receptor (TCR) therapies. And look for more technical discussions in BPI’s regular issue theme of cell and gene therapies in May of this year.
References
1 Rowley J. FDA Declines to Approve Enzyvant Regenerative Therapy on Manufacturing Concerns. LinkedIn post, 5 December 2019; https://www.linkedin.com/posts/jonrowley_fda-declines-to-approve-enzyvant-regenerative-activity-6608706565841178624-_BPF.
2 Hodge C. Industry Voices: What Can Reduce the Cost of CAR-T Therapy? Next Generation Therapeutics, 4 November 2019; https://knect365.com/next-generation-therapeutics/article/c3b619c2-0284-4ad7-ae40-2df0ae922164/reduce-the-cost-of-car-t-therapy.
Cheryl Scott is cofounder and senior technical editor of BioProcess International, PO Box 70, Dexter, OR 97431; 1-646-957-8879; [email protected].









