Voices of Biotech
Podcast: MilliporeSigma says education vital to creating unbreakable chain for sustainability
MilliporeSigma discusses the importance of people, education, and the benefits of embracing discomfort to bolster sustainability efforts.

In the April 2023 issue of BPI, Leonardo Ferreira discussed the biology of type 1 diabetes and how he has worked toward developing a cure for that disease at his laboratory at the Medical University of South Carolina using chimeric antigen receptor (CAR) T regulatory cells (Tregs) (1). He spoke about industry-wide advances in developing Treg technology and how lessons learned during preclinical trials can be applied in human trials. He also discussed what the industry needs to develop Treg advanced therapies successfully. We conclude our discussion here in Part 2.
The Advantages of Tregs
Why is your team researching advanced therapies based on CAR Tregs instead of CAR-bearing T effector cells or even monoclonal antibodies (MAbs)? How would Tregs treat type 1 diabetes? There is a lot of evidence supporting CAR T cells for advanced therapies. The US Food and Drug Administration (FDA) has approved several CAR T-cell therapies — for example, CD19 CAR T cells and B-cell maturation antigen (BCMA) CAR T cells for liquid tumors. Those types of products show that T cells can be purified from a person’s blood, genetically modified, and then redirected toward a new target. Of course, there can be complications such as cytokine storms and other side effects, but positive outcomes are possible.
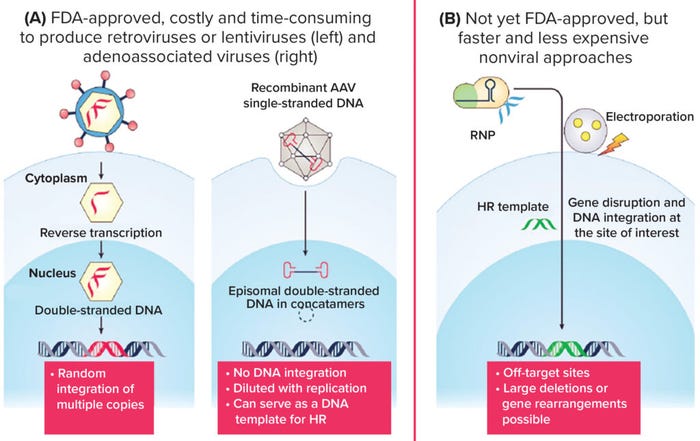
Figure 1: How best to genetically modify human Tregs? (HR = homologous recombination).
At Advanced Therapies Week (ATW) 2023 in Miami, FL, two speakers discussed using CD19 CAR T cells for systemic lupus erythematosus (SLE), which is caused by B cells. Depleting B cells is an unwanted side effect for cancer patients but is beneficial for lupus patients. Type 1 diabetes is a complicated disease because it’s in a person’s tissues. SLE is a systemic disease that can be treated by depleting cells throughout a body. With type 1 diabetes, action must be confined to the pancreas. You want a modality that will act only within the pancreas and pancreatic draining lymph node, so you need to consider how T cells kill and how to direct them. That might be possible if you could identify killer
T cells and make a receptor to recognize them, but doing so poses a challenge. There are 3,200 different human leukocyte antigen (HLA) alleles and more T-cell receptors (TCRs) in a person’s body than there are stars in the Milky Way galaxy. It would be hard to make a conventional CAR T cell to perform targeted killing for type 1 diabetes. So instead of killing the offending T cells, can we prevent their killing of islets?
Tregs are tissue resident, antigen specific, and provide a dominant form of immune tolerance. It would be ideal to make Tregs that can enter the pancreas and stop inflammation only in that organ. Unfortunately, they are rare among white blood cells, making up just ~1% of peripheral blood mononuclear cells (PBMCs). Tregs also expand slowly compared with conventional T cells, so you need many more cells to start with. Despite those difficulties, working with them is worthwhile because of their complete set of properties and functions.
Tregs have many mechanisms to stop immune responses. In addition to making cytokines such as interleukin (IL)-10, IL-35, and transforming growth factor (TGF)-β, they have CD25, which is a high-affinity IL-2 receptor alpha chain. IL-2–expressing T cells need to grow and proliferate, so immune responses are prevented when Tregs soak up IL-2. Tregs also have cytotoxic T-lymphocyte–associated protein 4 (CTLA-4), which is a receptor that binds to antigen-presenting cells (APCs) and blocks costimulation. They also have the ectoenzymes CD39 and CD73, which convert floating adenosine triphosphate (ATP) into adenosine (a potent immunosuppressive), acting directly on T cells to stop them from killing beta cells. As living drugs, Tregs are more powerful than any other small molecule or proteins you could use.
Using antibodies to treat type 1 diabetes is difficult because when they enter a body, they will spread indiscriminately until they bind to something. We can consider getting a bispecific antibody to bring Tregs together with killer T cells, but that would require finding surface markers present on Tregs that are not on conventional T cells. That could be prohibitively difficult because Tregs are a subset of T cells. There are always some side effects from blanket approaches, and that’s why we focus on regulatory T cells instead of conventional T cells or antibodies.
Our staff’s impression is that CAR Tregs would serve as a first step in a treatment regimen, coming before surgical implantation of pancreatic islets. How might CAR Tregs enhance that established procedure? To establish immune tolerance, you need to have fewer effector cells and more regulatory cells. For type 1 diabetes, you need both to make more beta cells and protect them. It’s important to consider whether it’s best to administer Tregs before or after surgery. Having Tregs in place beforehand could be beneficial, but we don’t know how they might distribute themselves around a body without any beta cells in place for them to recognize. We’ve shown in a mouse model that without a targeting mechanism, administered Tregs disappear quickly. But when CAR Tregs are injected intravenously, they will enter the spleen and then the islet transplant, where they stay and accumulate because the transplanted material provides the necessary activation signals for Treg survival.
On the other hand, if Tregs are administered too late, conventional T cells already will be detecting and attacking the new beta cells. It might then be best to bring the organ and the CAR Tregs in simultaneously. That way, the transplant organ comes in protected by a sheath of CAR Tregs that can stop an attack right away.
For that therapy to work most effectively, it should come in combination with effector T cell depletion. Alloreactive T cells can be present at a rate of 5% or more, so if 5% of your T cells attack the new transplant, then that number could be more than the implanted Tregs could handle on their own. Some companies that focus on Tregs use depleting agents against conventional T cells. Depleting conventional T cells makes it easier for Tregs to protect an implanted organ.
That also allows for target flexibility. We’ve worked on HLA serotype A2 (HLA‑A2), which is a specific allele in 30% of the population. In a transplant setting, you can have a donor who is HLA-A2+ and a recipient who is HLA-A2–. In most clinical settings, you would not want such an HLA mismatch, but in this case, the mismatch would raise a valid target that is present only in a transplanted organ.
If you make anti-HLA-A2 CAR Tregs, they will recognize only HLA-A2+ islets or beta cells. That specificity gives them an advantage over blanket treatments. One company, Sangamo Therapeutics, uses such technology to treat patients undergoing kidney transplants. CAR Tregs help to prevent rejection of the transplanted kidney in cases with an HLA-A2+ donor and an HLA-A2– recipient.
The Importance of Design
What factors do you need to consider to design an effective CAR? Because CARs are not present in nature, they have many moving parts that you need to research in a laboratory. CARs have an antibody-based binding domain called a single-chain fragment variable (scFv) that gives them specificity. We made an scFv for HLA-A2 designed for a cell surface.
An scFv has a hinge that connects it to an internal transmembrane domain. The hinge remains outside a cell, rather than inside the membrane. The intracellular domain, usually a CD28, determines how a cell behaves. The scFv, hinge, transmembrane, and intracellular domain are amenable to optimization. The physical connection between the hinge and transmembrane domain affects how a cell signals and binds.
With type 1 diabetes, the CAR Tregs that we use are unlike conventional T cells. The signaling that optimizes T-cell killing, inflammatory cytokine secretion, and migration doesn’t necessarily optimize Treg suppression, antiinflammatory cytokine secretion, or binding to APCs. We are seeking to retailor CAR signaling for Tregs, and because we want opposite functionality, we don’t suspect that the signaling will be the same.
Conventional T cells thrive on phosphoinositide (PI) 3 kinase, protein kinase B (AKT), and mammalian target of rapamycin (mTOR), whereas Tregs do not. If you add rapamycin to a mix of Tregs and conventional T cells, it will inhibit the PI3 kinase, AKT, and mTOR. Conventional T cells will stop growing and die, whereas the Tregs survive just fine. Rapamycin has immunoregulatory effects that promote Tregs to the detriment of conventional T cells.
Different molecules will have different effects on Tregs, meaning that it’s also important to consider other drugs that patients are using alongside treatment with Tregs. If, for example, a patient takes steroids, low-dose IL-2, or rapamycin, then those molecules can interact with CAR Tregs. There is a whole complex field of interactions between engineered cells and other drugs. Such considerations are important because they affect how we design new CARs.
In highly inflammatory environments, Tregs can transform into T cells, which is a problem you need to watch out for. You don’t want to inject your beautifully engineered Treg into a patient to stop inflammation only to see it turn into an effector cell. Not only must we concern ourselves with making enough Tregs for therapeutic application, but we must also work on keeping those cells stable.
Aside from determining a CAR’s binding domain and drug interactions, affinity is also important. T-cell receptors usually have lower affinity than antibodies do. In this case, we are forcing Tregs to act with a CAR instead of with a T-cell receptor, resulting in a “Frankenstein” cell that sees the world as if it were a B cell.
How can human Treg cells be genetically modified to express CARs? What advantages and disadvantages come with viral and nonviral approaches to gene delivery? For research and development (R&D), our two main methods to modify human cells are lentiviral transduction and gene knock-in mediated by clustered regularly interspaced short palindromic repeats and associated protein 9 (CRISPR/Cas9). For both methods, we purify Tregs from a person’s blood, then activate and modify them.
We use HEK293 cells to produce lentiviruses. It is a straightforward process but subject to a manufacturing bottleneck as we try to get supplies into our clinic. Lentiviruses bind to cells, then enter cell nuclei and integrate into their genome. That integration is key because every time Tregs divide, the daughter cells will maintain their original construct. On the downside, integration is random. If a cell integrates with a tumor-suppressor gene, for example, then undesired cells could result.
You also need to compare the numbers of viral particles with the number of cells. Ideally, you want to attain one viral copy per cell. If, for example, one cell has 10 copies of a virus, then it will express much more CAR protein than would a cell with only one copy. That results in an unpredictable, undesirable product. Ideally, you want every cell to have one copy of the virus in a cell suspension, but that’s hard to control.
We are working constantly on new virus modifications. We work with new CARs and signaling domains in our laboratory every week. Viral methods are convenient and will continue to have value in preclinical settings. For preclinical testing, they work well. For actual patients, nonviral methods are safe and reliable. With CRISPR/Cas9, scientists use a knock-in method to target precise genetic locations at one copy per cell. Thus, the method does not rely on random integration. When you use Cas9 as a nuclease, guide RNA tells cells where to target the endogenous TCR locus. They then make a double-strand DNA break and add a gene of interest to repair the DNA using the sister chromatid.
The cell is tricked into repairing its DNA not with an endogenous sister chromatid, but with the homology-directed repair (HDR) template administered by scientists. This template is inside a CAR. After you make a double-strand break in a cell, it repairs its DNA by sorting the HLA-A2 CAR into the T-cell locus, effectively replacing a TCR with a CAR.
That is advantageous over simply dropping a CAR into the genome with a still-present TCR that can cause side effects and be recognized by allogeneic T cells. By replacing a TCR with a CAR, you ensure one CAR copy per cell. The TCR promoter and enhancer are there, so when a T cell is activated, you can achieve maximum CAR expression. If you have too much or too little signaling, T cells can help by regulating expression levels. On the other hand, using lentivirus can cause donor-to-donor variability. Even if you apply the same amount of virus, cells from different people provide different percentages of transfection efficiency.
With knock-in methods, patient variability is minimized. Similar percentages of CAR+ cells will arise because of the precise engineering performed with a CRISPR/Cas9 and HDR template. You transiently open pores in the Treg membrane and insert Cas9 protein and guide RNA. Then you add an HDR template to the cells using adenoassociated virus (AAV), which is a special type of virus that does not integrate into the genome. We use electroporation, but that can be hard on cells. We are working to improve that process by using hydroporation instead, which gently deforms cells instead of zapping them with electricity. Modifying Tregs is an interesting and challenging process because they are less sturdy than conventional T cells and more prone to changing their phenotype. And again, if you push a Treg hard enough, it will become an effector T cell.
We continue to research how to modify Tregs, and doing so is a subject of ongoing interest in the field. There were booths at ATW 2023 about how best to modify T cells and Tregs for immunotherapy. When I was a postdoctoral fellow at the University of California San Francisco (UCSF), there were patients who we could not be infused with Tregs because their cells wouldn’t expand enough. Many factors matter with improving cell modification, from how many cells you have to how well they are able to expand. It’s important to understand how to keep CAR Tregs as happy as possible. It’s not just efficiency that has to be optimized, but also the viability of the cells.
What can you tell us about the CAR Treg expansion? How does CAR Treg production work, and what elements are required? First, you need to get the Tregs for CAR Treg expansion, usually by isolating them from peripheral blood using fluorescence-activated cell sorting (FACS). Using FACS tends to provide better cell purity than other methods such as magnetic-activated cell sorting (MACS), and purity is especially important for Treg production.
At ATW 2023, Miltenyi Biotec exhibited a MACS tidal FACS system, which solved a sterility problem for FACS by introducing single-use cartridges. Usually FACS systems become nonsterile because of how samples go through the machines. No matter how many times you run bleach through your system, you can’t ensure total sterilization. Collecting Tregs in single-use cartridges solves that problem.
In most trials, you extract Tregs from blood using a combination of MACS and FACS. You can use magnetic enrichment of CD4 T cells, which can raise the percentage of Tregs in collected blood from 1% to 5%. You can use CD25 enrichment, which could raise that percentage all the way to 70%. You also can filter out cells based on what they don’t have. Tregs are CD25 high, but they are also CD127 low. You can increase purity by excluding some activated T cells that could contaminate your sample.
The most popular method is to activate Tregs using magnetic beads coated with anti-CD3 and anti-CD28 MAbs. Putting those beads into cells simulates an infection. Tregs will begin to cluster in response to a perceived infection. Cells that used to be round become elongated and start to expand.
The activation levels of Tregs in a culture will go up and down, and you must let them go back to a resting state before forcing them to activate and divide. If you activate Tregs too quickly in succession, they will experience activation-induced cell death (AICD). You need to find the optimal timing to restimulate them.
For example, if you begin by activating a sample of 10 million Tregs, you want to let them grow for nine days, allowing the culture to plateau. On day nine, you activate them again, and then let them go until day 14. Over the course of two weeks, you activate them twice. In such a case, they could increase from 10 million cells to 200 million cells. From there, you can get your desired 1 billion or even 5 billion cells after a second run of stimulation.
Of course, the last thing you want is to inject a patient with T-cell–activating beads, so we remove those from a bag of cells using a big magnet. Some cells refuse to let go of the beads, resulting in some loss, but the field is making gains in that area. There are new activation methods that don’t require removal because they are soluble and designed to degrade over time.
The field also has experienced gains through cell culture media and component advances as researchers explore what cytokines and growth factors to add. For human therapeutics, cell culture media should be chemically derived and free of animal-derived components. IL-2 is mandatory for Tregs to live, but they don’t make it themselves. So it must be added. We also found that adding IL-6 and tumor necrosis factor alpha (TNF-α) to a pure Treg culture allows for better Treg growth and expansion. That surprised us because those are inflammatory molecules.
But even if you’re able to make 10x more cells, Tregs are worthwhile only if they maintain potency — and from our observation, they did. Mouse models show that if you inject the same number of Tregs, grown either conventionally or with extra cytokines, they work fine. That discovery is promising because it affects the cancer field as well. Some cancer patients never get their CAR-T dose because the cells fail to grow enough. Cell expansion is an issue for conventional CAR T cells but an even greater issue for CAR Tregs because of their rarity and slower rate of expansion.
Of course, you want to genetically modify CAR Tregs at their peak of activation, when cells are at their biggest size and their nuclear membranes are recycling and regenerating. At that point you can either perform two rounds of activation using magnetic beads, or you can try to be clever by finding a way to activate only CAR Tregs.
Such a process would involve depleting CAR– cells and selectively expanding CAR+ cells. For example, you could have HLA-A2–expressing irradiated target cells, and then you have your HLA-A2 CAR Tregs, some of which have had that allele knocked in. During the second round of activation, the HLA-A2 Tregs will become stimulated and expand exponentially, whereas the unmodified Tregs will die out. You also could use purification. Although magnetic sorting struggles with purity, going from 60% to 100% (for example) isn’t difficult because that is less than a twofold enrichment. You can’t go from 1% to 100% modified Tregs, but you can get dramatic improvement when starting from a higher percentage. That enables you to bind your cells of interest or deplete those that you don’t want.
You can deplete the TCR-expressing cells that still have their TCRs. By doing that, the only cells left behind are those that don’t have that receptor, which happen to be those with the CAR knocked in. If you want an untouched enrichment of cells, then you can leave your desired cells alone and weed out the undesired ones. The method you choose will depend on the cell mixture that you have and which specific disease or indication you’re targeting.
Whether to optimize stability and viability or efficiency is a debate that has gone on for decades, and discussions about enriching cell populations are similar. Do you do direct enrichment for positive selection, or do you deplete the material that you don’t want and leave your desirable cells alone? Maybe you can do both.
Reference
1 Abbott J, Ferreira L. Using Regulatory T Cells for Treatment of Type 1 Diabetes, Part 1. BioProcess Int. 21(4) 2023: 10–12.
Josh Abbott is associate editor of BioProcess International; [email protected]. Leonardo Ferreira, PhD, is an assistant professor of microbiology and immunology at the Medical University of South Carolina; [email protected].
You May Also Like