 Assay lifecycle development for traditional biopharmaceuticals such as vaccines and monoclonal antibodies (MAbs) has a clearly defined pathway, from preclinical method selection, development, and optimization through the milestones in preclinical phase trials, and finally to postlicensure method evaluations, comparability, and improvements. The analytical development roadmap for nontraditional biologics such as chimeric antigen receptor (CAR) T-cell therapies and gene therapies are not as clearly defined and can present many challenges along the way. Understanding the “what, how, and when” of analytical development for CAR T-cell products can help developers of such therapies find solutions to those problems. Andrea R. Moore, director of analytical development at Tmunity Therapeutics presented on the analytical testing strategies for these products at the BioProduction Congress in June 2019. This article is based on my discussion with her about that presentation.
Assay lifecycle development for traditional biopharmaceuticals such as vaccines and monoclonal antibodies (MAbs) has a clearly defined pathway, from preclinical method selection, development, and optimization through the milestones in preclinical phase trials, and finally to postlicensure method evaluations, comparability, and improvements. The analytical development roadmap for nontraditional biologics such as chimeric antigen receptor (CAR) T-cell therapies and gene therapies are not as clearly defined and can present many challenges along the way. Understanding the “what, how, and when” of analytical development for CAR T-cell products can help developers of such therapies find solutions to those problems. Andrea R. Moore, director of analytical development at Tmunity Therapeutics presented on the analytical testing strategies for these products at the BioProduction Congress in June 2019. This article is based on my discussion with her about that presentation.

Figure 1: Traditional assay lifecycle development has a clearly defined roadmap.
Analytical Development Challenges
Figure 1 shows the typical analytical development lifecycle for well-established biopharmaceuticals. In such cases, it is clear what testing is required at each phase. However, analytical development for CAR T-cell products and other nontraditional biopharmaceuticals do not always follow such a roadmap. Potential challenges include patient sample variability, small lot sizes, potency needs, and product stability concerns. Small lot sizes are problematic because analysts have only a small amount of material to use for conducting many tests. Risk-balanced development is another challenge. “As you’re developing analytical methods, you invest time and money into needed products without knowing whether they are effective,” says Moore. “On the other hand, if you don’t invest that time and money for those tests and the product moves to a pivotal trial, you’re already behind.”
Some biopharmaceuticals such as autologous cell therapies require rapid release, and analysts must adjust testing methods to meet short timelines. “You have to get creative [about] how you’re releasing [CAR-T products] because the path isn’t clearly defined,” says Moore. Some drug products will go to phase 1 after the preclinical stage, which allows for additional assay development time, but a blockbuster drug might go directly to pivotal trial and development, shortening the available time frame for analysis, with no opportunity to progress through several clinical trials.
Management Areas: In her presentation, Moore highlighted three areas of focus questioning “what, how, and when” in regard to managing the development and release of CAR T-cell products:
Release Testing: What should be in the assay panel?
Qualification and Validation: How and when should they be conducted?
Reference Standards and Controls: How are they defined and controlled?
The “Release Panel” box lists a typical release panel for most CART T-cell products. “Because of the small lot size of these products and the need for rapid release testing, we try to keep those tests simple,” says Moore.
Most scientists understand the difference between qualified and validated assays but might be unsure about when to perform them. Moore says that methods are qualified typically during early clinical phases. Full validation is performed “at the point where processes are locked, and product is in its final matrix. That is because validation is matrix dependent. Defining those two points is important upfront, so you know when you have to push for validation. Method validation itself should be conducted by following a traditional pathway and regulatory guidances” (see the “Method Validation” box).
Although validation for CAR T-cell therapies and other emerging therapies might take more time and creativity than for other therapies, it still needs to be done. Moore says she has conducted validation for many different assays, but emphasizes that each product is different. “How we address challenges requires valid analytical justification because CAR T-cell and other cell therapies are held to the same standard as other pharmaceutical products when it comes to adherence to regulatory guidances.” If you can’t, for example, report a certain parameter in an assay, then you need to justify why it can’t be reported and how you’ll mitigate the absence of those data. A thorough understanding of the methods and how to use them to obtain all the necessary data information are critical.
Using the best material for reference standards and controls is essential for understanding method performance. That is especially true for autologous cell and gene therapies. “It’s not as easy as making one large lot of material and determining that everything produced the same way as that lot will perform the same way as that material. Every patient cell is different. For autologous products, nothing is off the shelf,” says Moore. Cell and gene therapy products are too specific for commercial reference standards, so standards are generated in-house and based on a limited supply of product.
Defining assays and being able to control them also can be difficult. Some methods have more standardized controls than others. With flow cytometry, for example, standardization of critical materials, such as isotype controls and beads now is more established than it was five to 10 years ago. “Standardization of controls has been moving in the right direction, but confirmation of performance of those materials still is needed because of patient variability. Despite some standardization, certain assays will still require additional customized assay controls.”
Flow Cytometry Case Study
In her presentation, Moore used the flow cytometry assay as an example of some challenges that needed to be overcome when developing an assay. She presented three major areas on which to focus: defining assay requirements, procuring equipment, and defining protocols. Determining assay requirements starts with defining the assay’s purpose, including critical parameters to be measured and whether the assay will be part of the product release or used solely for further characterization.
Equipment procurement involves determining how many parameters need to be tested and defining sample format and volume needs. “For flow cytometry testing, the FDA has focused on data integrity and validation. Understanding, for example, what gating strategies were used and clearly defining an audit trail are important. You also need to take into account how many parameters you’re testing. For flow equipment, that depends on the equipment — whether, for example, you’re testing four colors, 10 colors, or 16 colors.” Equipment compliance, installation qualification (IQ), and operational qualification (OQ) also are part of this area. “A lot of equipment is only for research use, so you have to take time to understand your equipment.”
Defining protocols involves evaluating assay variables step by step and establishing equipment standard operating procedures (SOPs). Moore advises starting with a general protocol, but as you choose your reagents and understand critical parameters, adjust that protocol according to the variabilities you find. Her presentation highlighted some method variables to be evaluated for flow cytometry. They include
incubation time and temperatures
Moore presented an example of how reagent selection influences sensitivity. She highlighted results from a study of an antibody conjugated with three different fluorophores (phycoerythrin, fluorescein isothiocyanate, and allophycocyanin) to stain the same sample. The percentage of single-chain variable fragment antibody (scFv) signal varied from 24% to 59% at 729 dilution and from 7% to 28% at 2,187 dilution (data not shown). When developing flow cytometry methods, Moore advises “balancing reagent selection considering the cells and the specifc markers expressed on those cells, for example, by using a dimmer fluorophore for a marker that’s highly expressed to prevent washing out the signal or using a brighter fluorophore with a marker that is expect to be expressed rarely in an effort to enhance a signal.”
Optimization of the staining procedure for flow cytometry is another source of method variability to be evaluated. Factors to consider include cell wash temperature (cold, warm, or room temperature), blocking steps, staining buffers, antibody concentration, and incubation conditions.
Ensuring that critical reagents used in assays are controlled and their performance is understood provides insight into data interpretation as process development occurs. If lot-to-lot variability of an antibody has been observed in a flow assay, new lots received by a laboratory can undergo titration assays before use to confirm appropriate dilution during routine testing. Another alternative is to procure large antibody lots. However, assessment of their long-term stability would be required. “This control is important, especially during method development, which necessitates a clear understanding of any observed changes in data so that an accurate assessment can be made to determine if those changes are from changes to process or assay variability.”
Moore’s case study offered an example of the need to focus on the big areas first, using experience to address the most troublesome variables and ensure that all critical parameters of the assay are under control.
After Method Development
Much work needs to be done after method development. Moore suggests continuous improvement of methods to understand how they are working and how data are trending. “Much of that refers back to having controls. If you don’t have controls, you have no idea what’s going on, and you don’t know how to improve.”
As clinical trials move from phase 1 to phase 3, methods must be refined and optimized toward the goal of being specific to the individual program. Figure 2 shows areas to consider during optimization of a method.
For flow cytometry, developing product-specific reagents includes the following:
Peptides or antibodies are labelled for flow detection.
Stability and control are necessary but take time.
Product-specific reagents help in identification of products in a multiproduct facility.
Generation can be time-consuming and costly.
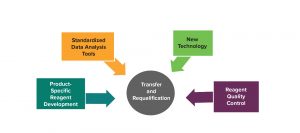
Figure 2: Continuous improvement after assay method development
For some products expressing chimeric antigen receptors, no commercially available reagents exist that can detect them with required specificity. Some generic reagents can be used initially, but not in every case. “That is, again, a balance between committing time and resources to making such product-specific reagents without putting my trial at a detriment. Keeping the balance between your need for product-specific reagents and faster timelines is important.”
Moore also highlighted the benefits of using standardized data analysis tools, including
predefined instrument settings and acquisition
automated gating built upon a significant amount of data to build algorithms.
comprehensive understanding and procedure for gating strategies.
Currently, especially for flow cytometry, analysts obtain their data and then need to perform gating manually. “There is a push now with some equipment and software suppliers to help automate this process to eliminate human variability,” says Moore. “If the process is performed manually, you might put a line just to the right and change a percentage slightly, but if that happens multiple times a little more over time, you could see a big difference. So automating the gating process would help reduce those errors.”
Integration of new technology proceeds along the same lines. Some attempts have been made to automate flow cytometry (cartridge technology), for example, although that will take some time and development. Full automation of a flow cytometry method will require ensuring that panels and gating strategies are defined clearly for the many samples to be analyzed. New technology can improve equipment sensitivity and can be used with newly developed reagents.
Reagent quality control often is overlooked. A critical reagent control and qualification program can proceduralize the storage, use, and inventory management of these materials, thus providing additional insight into reagent stability and helping analysts tighten specifications with data collection over time.
Assay Validation
Validation is required for pivotal studies. Moore suggests that analysts work with their clinical teams, and a solid interdisciplinary team is needed to know when the time is right to validate. “Keep in mind that you can perform the same assays repeatedly throughout development and qualification. Eventually, you reach a point at which you are comfortable with how specifications have been set. At that time, you validate and move on. That doesn’t mean you can’t go back. New technologies will continue to improve the way testing is done. Assay validation is a continuous process. You can decide to make changes that are controlled and justified along the way.”
During her presentation, Moore pointed out that no special guidelines exist for CAR T-cell products. Analysts need to take strategic approaches about how to validate assays for such products considering intra- and interassay variabilities. Evaluation guidelines also must be followed. Some parameters can’t be validated (e.g., linearity and accuracy may not be applicable for flow validation activities). But those decisions should be justified.
An Evolving Approach
Assay development and improvement for CAR T-cell and other cell and gene therapies is a continuous process. “You can platform as much as you can, but that might never work out for these new therapies. With CAR T-cell and other autologous therapies, each patient is different, so each product is different. It’s essential to understand what you can and cannot control. It all goes back to understanding your assay and how it’s going to perform under certain circumstances. That’s also why having control is essential.”
Clinical development needs to be in lockstep with chemistry, manufacturing, and controls (CMC) to expedite timelines efficiently. “Timing is important because some patients go through lymphodepletion before receiving treatment. Working closely with your clinical team is important to understanding when a product is made and when it is scheduled for release. It’s a huge logistical challenge, especially when you are working with multiple clinical sites where patients will receive infusions. Critical to the logistics is having a chain of identity that starts the moment cells leave a patient to the moment those cells are returned to ensure that patients receive their own cells.”
Cell therapy methods are inherently challenging and require new ways of thinking. “There aren’t compliance exceptions for CAR T-cell products. You need to take some creative approaches in how to strategize your analytical landscape,” says Moore. “You have to run many tests for every patient. Keep it simple at the beginning. You can go through a large amount of testing before a product is released. If you try to do too much for one batch of an autologous cell therapy, you could run into a problem of not having material left or with assays taking too long. Meanwhile patients are waiting, so the faster you can get them their product, the better. That’s one of the reasons why I like what I do. Every day is a new challenge.”
Maribel Rios is managing editor at BioProcess International; [email protected]; Andrea R. Moore is director of analytical development at Tmunity Therapeutics Inc. 3020 Market Street, Suite 535, Philadelphia, PA 19104; 1-215-966-1600.










