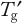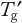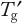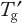Production of biologics is expensive. To optimize capacity use, bulk protein solution produced in manufacturing campaigns is often converted into drug product based on market demand, so it may be stored for relatively long periods. To decouple production of bulk solution from that of a final drug product, the bulk is often stored frozen.
Transport of frozen bulk between sites offers several practical advantages over bulk transport in the liquid state (2–8 °C). Maintaining 2–8 °C requires accurate systems control to ensure that bulk product does not get too cold and (partially) freeze. A liquid shipment also subjects proteins to shear and agitation stress at air–liquid interfaces. A successful bulk storage program therefore enhances bioprocess capacity use and reduces overall production costs. But success requires careful consideration of biophysical and engineering principles in the development of a frozen storage operation and its effects on products.
PRODUCT FOCUS: BIOLOGICS
PROCESS FOCUS: DOWNSTREAM PROCESSING, FORMULATIONS
WHO SHOULD READ: QA/QC, PROCESS DEVELOPMENT, MANUFACTURING, AND FORMULATORS
KEYWORDS: Disposables, excipients, container–closures, storage, bulk intermediates, transportation
LEVEL: INTERMEDIATE
In Part 1, we reviewed the fundamental aspects that determine the behavior and outcome of protein freezing (1). Here in part 2, we examine some technologies available for large-scale freezing and storage and provide guidance on rational development of formulations and processes for this unit operation. An abridged version of this review was published elsewhere (2).
Bulk Freezing and Storage
Freezing of biologics at large scale can involve improvised, catalog-purchased, or purpose-designed systems. The choice is often dictated by the volume to be frozen, available technology, shipment needs, stability of the frozen matrix, and so on. The simplest storage concept involves filling bulk solution into bottles/carboys of appropriate size and storing them in freezers. Such containers are often made of polyethylene or polypropylene, although 316L stainless steel can be used for small volumes.
The advantage of this system is its simplicity. Disadvantages include a lack of active process control and potential variability between containers as well as multiple container–closures that must be secured against contamination. Loading a number of containers filled at room temperature into a freezer can easily overwhelm its cooling capacity, leading to long and variable freezing times among those containers. Therefore, parameters must be well defined, qualified, and validated: e.g., preconditioning of both freezer and containers, fill volumes and loading patterns, and maximum and minimum loads.
Thawing is generally performed by placing containers in a refrigerator or at room temperature. In the absence of an active mechanism, thaw times can be quite long (possibly days) depending on container size. During this period, significant concentration and temperature gradients will exist in each container if they are not actively shaken/agitated (3), as in Figures 4A and 4B in Part 1 (1). Practical handling considerations limit the size to ~20-L carboys, although 50-L sizes are possible. However, the bottle/carboy system is simple, and if a protein in formulation is robust and stable under a wide range of freeze–thaw conditions and can withstand cryoconcentration, this is the preferred mode of operation for many companies.
Variations intended to overcome the passive freezing limitation above include blast cooling with (vaporized) liquid nitrogen and dry ice/ethanol baths. But we are unaware of published studies on the freezing of bulk-scale proteins with such systems.
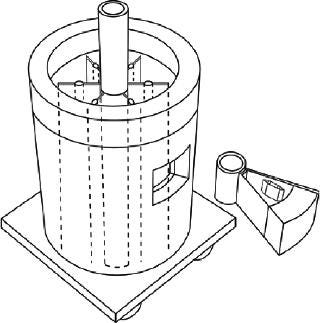
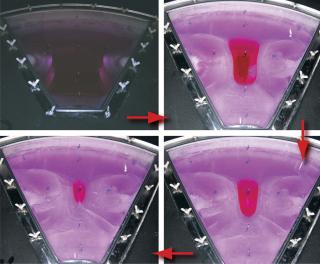
Sartorius-Stedim (www.sartorius-stedim.com) offers a large-scale bulk solution for freezing in stainless steel vessels (cryovessels). Fundamental and practical aspects of the system design have been published (4). These vessels consist of jacketed, cylindrical, stainless steel tanks each with an internal, radial, finned, heat exchanger that effectively divides the tank into eight longitudinal sections. That reduces the heat-transfer distance and improves heat transfer across the entire volume (Figure 5). This CryoFin technology is said to promote dendritic ice formation, thus avoiding potentially damaging effects of cryoconcentration (5,6,7). Cryoconcentration is unavoidable, however, given the distances involved for heat and mass transfer (Figure 6) (8).
Figure 5: ()
Figure 6: ()
Cryovessels are cooled and heated by an external refrigeration system that circulates heat transfer fluid through the jacket and core of the fin system. The temperature profile of that fluid is programmable for reproducible vessel temperature profiles. The vessels are kept stationary through freezing below 0 °C, but they are gently agitated by rocking during thawing. The lack of agitation during freezing prevents solute movement and promotes dendritic ice formation. Agitation during thawing, however, promotes rapid mixing of thawed material, thereby removing concentration hot spots and maintaining a uniform temperature in the solution for rapid thawing. The lowest working temperature for this equipment is −60 °C, and nominal vessel volumes range 20–300-L.
One variation of bulk-freezing technology is the FreezeContainer system from Zeta Holdings (www.zeta.com). Jacketed vessels (currently limited to 300-L) are cooled or heated through an internal circulation system (mounted in their lids). Heat exchange is accomplished by an external refrigeration system through a circulating heat transfer fluid. The temperature profile is programmable, and the entire container is agitated during thawing.
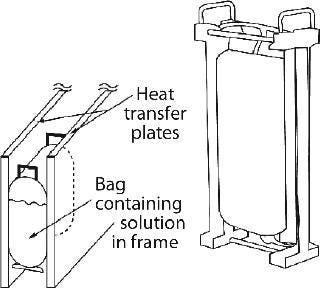
Sartorius-Stedim also offers Celsius large-scale bag freezing systems using upright bags made of Stedim71 film (ethylene vinyl acetate product-contact material). These are filled with a solution to be frozen and held with slight compression between two plates that serve as heat-exchange surfaces. The plates are cooled/heated by circulating heat transfer fluid from an external, programmable refrigeration unit. The slight compression provides improved contact and heat transfer, resulting in a frozen bag the shape of a pillow (Figure 7). The bag is placed in a frame so as not to damage the material inside during handling and transport. Currently available nominal bag sizes are 8.3 L and 16.6 L, with fill volumes ranging 2.1–8.3 L and 4.2–16 L, respectively. Six bags can be simultaneously processed in one cryo unit for a combined maximum total volume of 100 L. The bags are kept stationary during freezing, but the whole unit is rocked during thaw to promote mixing in the bags. Further mixing of the thawed solution is required, however, to ensure homogeneity of the bag’s contents.
Figure 7: ()
Developing Frozen Storage Options
Formulation development for proteins has traditionally focused on products and their final presentation. Bulk storage has been considered as an afterthought. For a proactive approach to this step, some practical guidelines are provided here. Note that a composition optimized for frozen bulk storage may not be the same composition as that of a marketed drug product. In general, the excipients used for frozen bulk storage will be the same or a subset of those of the drug product. However, their concentrations may be different.
Excipients for Frozen Storage Stability: The following list provides a starting point for identification of a formulation with appropriate protective properties for the studies suggested here: Sugars, polyhydric alcohols, certain amino acids, and salts (as well as polyethylene glycol, PEG) have cryoprotective properties. In addition, a surfactant may be required to protect against ice-interface–induced denaturation, although PEG has been proposed to protect by the same mechanism.
Freeze–Thaw Cycle Stress Studies and Formulation Development: Based on a knowledge of the phenomena occurring during freezing and thawing, it is generally considered best to do both as rapidly as possible to reduce time spent in transition (1). Because of their size, however, production systems cannot be processed as rapidly as small-scale systems in laboratories. Performing freeze–thaw (FT) cycle studies is standard practice in formulation development to assess the ability of an excipient to provide thermodynamic and interfacial stabilization. FT studies therefore should be designed to cover a range of cooling and heating rates.
Depending on volume, freezing with a significant degree of supercooling and rapid nucleation, slow freeze, and slow thaw without agitation provide the greatest stress. FT cycles should cover a range of temperatures, but thawing/holding between −10 °C and 5 °C are likely to be worst case because it prolongs time spent in transition. If the primary mechanism of FT-induced structural damage is denaturation at the ice interface (1), studies at low biologic concentrations are more likely to produce detectable changes.
If cryoconcentration-induced changes in solute environment (as described in Part 1) are the main mechanism of damage, then a high-concentration formulation may be the worst case to test. Thus, the impact of FT cycling should be evaluated at both low (~1/3×) and high (~3×) protein concentration levels. These studies can be used to identify which excipients function best as stabilizers and will likely include excipients that function through preferential exclusion and through prevention of unfolding at the ice interface. Excipients that crystallize on freezing are unlikely to be effective cryoprotectants.
Follow-up studies should optimize the composition to move the glass transition temperature (
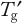
) toward higher temperatures (as discussed in Part 1). This generally requires increasing the protein concentration while decreasing the stabilizer concentration. The objective is to find the least amount of stabilizer required to provide adequate FT and frozen storage stability.
Appropriateness of a composition should be confirmed by longer-term stability studies. In many cases, this would make the composition of the frozen bulk different (higher protein, lower stabilizer concentrations) from that of a final drug product. However, the difference can be readily adjusted during final product manufacture with a suitable diluent. Crystallizing excipients will not provide a glass transition temperature.
Long-Term Frozen Storage Studies: As discussed in Part 1, practical storage conditions are preferably below
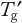
of the matrix unless a protein is robust enough to tolerate a higher temperature. Frozen-state stability studies that are above and below
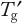
are required as part of formulation development. Prolonged frozen storage is a stress that unfortunately cannot be easily mimicked by an accelerated study. Freezer temperatures of −70 °C, −40 °C and −20 °C are common because most disaccharide cryoprotectants have glass transitions around −30 °C. A possible accelerated temperature is −10 °C. But to ensure that all stability samples are frozen, they should be first placed at −40 °C before stability testing. This is obviously most important for the −20 °C and −10 °C samples, which may not freeze otherwise. The US Pharmacopeia defines the −20 °C condition as ranging from −10 °C to −20 °C, and the European Pharmacopoeia defines this as −20 ± 5 °C, so the impact of temperature fluctuations must be assessed. Again, a −10 °C storage gives a worst-case condition.
Choice of Technology: For this discussion, we are calling systems that incorporate active cooling/heating (such as the cryovessels) as active, whereas passive systems consist of bottles/carboys placed in a freezer. At the outset, it must be recognized that commercially available active, controlled FT systems control only the rate of heat removal/supply. During freezing, that enables the reproducible application of a predefined rate of heat removal. During thawing, the ability to actively heat a frozen mass while providing agitation is an advantage that enables practical reduction of thawing time. However, despite being called “controlled,” such systems do not control the degree of supercooling, the rate and extent of ice nucleation, and the final ice-interfacial area achieved, all of which are critical to the ultimate outcome of a process. Controlled systems simply increase the probability that those parameters would be consistent from batch to batch.
The “FT Technology Considerations” box lists some factors that would come into play in selection of FT technology.
Process Development and Stability Studies
Scale-down models that mimic large-scale systems are useful for development studies and could also be used for regulatory stability studies. Selection of a technology for bulk protein storage affects the design and execution of ICH stability studies. ICH Q5C states that “drug substance entered into a stability program should be stored in containers that properly represent the actual holding containers used during manufacture.” Although traditionally the “representation” has been limited to construction materials, consideration from a process perspective also may be required. However, if a system is to be used for regulatory stability studies, it must be robust, qualifiable, and not too cumbersome to handle in a QC environment.
To obtain similar time–temperature profiles, scaling down should be performed on the basis of heat and mass transfer dimensions. This requirement makes it impossible to truly mimic a system of bottles/carboys using smaller containers. Given the lack of alternatives, general practice is to use small bottles/carboys of the same material of construction and simply place them in freezers to freeze (and in a refrigerator or at room temperature to thaw). The validity of those smaller-scale alternatives can be improved by measuring temperature profiles in the larger containers. Full-scale temperature profiles should be obtained in freezers and refrigerator/room temperature situations by placing carboys/bottles in “worst-case” locations from a heat-transfer perspective. Placebo solutions can be used to obtain such profiles. Resulting profiles can be mimicked to some extent in smaller bottles by placing them in programmable glycol baths for freezing/thawing. Other, less-exact alternatives include wrapping the smaller bottles in insulation to slow down their rate of cooling or heating when placed in a freezer or outside to thaw. But such handling is obviously cumbersome (with associated risk for deviations) and is seldom incorporated as part of formal stability studies.
FT TECHNOLOGY CONSIDERATIONS
Product Robustness: Passive systems will generate a greater degree of variation among containers of one batch (placed in different spots) and potentially between different batches. Such variability can be reduced by defining pretreatment, placement, and load. Active systems allow tighter process control. However, all parts of material in a tank or bag do not experience the same freezing process, so formulation must be designed to accommodate inherent variabilities.
Process Volume: Passive systems are limited by storage and handling considerations. Current knowledge suggests a 20-L maximum per carboy, although 50 L may be possible. Batch size and logistics determine selection. Active systems vary from 2.1 L (minimum bag volume) to 300 L (stainless steel tank).
Capital: Passive systems require appropriate freezer dimensioning. Consideration must be given to whether containers are disposable or reusable. Active systems require specialized equipment involving significant capital investment.
Material Compatibility: Passive systems imply availability of a variety of contact materials: Polyolefins (HDPE, PP), PETG, and stainless steel are common. Extractables and leachables — including the effects of reuse (e.g., repeated cleaning and sterilization cycles) — must be considered as a part of qualification of compatibility. Active systems are currently limited to stainless steel, hastalloy, or bags with ethylene vinyl acetate (EVA) contact layers. Again, appropriate compatibility studies must be performed.
Storage Temperature: Passive systems can be processed down to −70 °C or −80 °C and stored. Available freezers accommodate most sizes. For all plastics-based containers, due consideration of handling must be given to fragility at such temperatures. Most noncrystalline polymers have a glass transition temperature. Brittleness temperatures are published for a number of polymers (e.g., www.matweb.com). Active systems can process down to −50 °C in general, and operational storage for stainless steel systems is probably also limited to the same temperature. Bags can be stored at −70 °C or −80 °C with some handling issues due to possible fragility. Storage below −20 °C with either type of system requires special facilities because of the sizes and volumes involved.
Miscellaneous (Container–Closure Integrity, Maintenance, Shipping, and Process Validation): Passive systems may require multiple containers for storage of one batch of solution — so multiple container–closures would be involved, implying some risk of contamination. When disposable containers are used, their cost and disposal have to be addressed. If multiple-use containers are the choice, then their maintenance (including closures), cleaning (validation) program, and lifetime must be evaluated. For transportation, multiple containers increase contamination risk. Well-defined protocols must be created for handling, fill volumes, preconditioning, FT, placement, loads, and so on. In-process monitoring positions must be defined. A proper validation program will account for all possible variations.
Active systems using stainless steel vessels also require significant maintenance and cleaning (validation) programs. The disposable bag system is advantageous in this respect, but cost of disposal and waste management will have to be considered. Options are available for transportation. Such systems should be easier to validate because their process parameters are controlled and reproducible.
The Cryowedge scaled-down system for cryovessels represents one small wedge from the tank as Figure 5 shows. Critical heat-transfer dimensions of a full tank are mimicked in this system for studying process and formulation development at small-scale. The Cryowedge cooling/heating program can be designed to obtain temperature profiles in a wedge similar to what would be obtained in a full-scale tank. This scale-down system provides insight into large-scale freezing and thawing, and our studies show that it is not devoid of cryoconcentration effects.
The system is scaled down even further with a Cryocassette system, small 30-mL and 100-mL size cassettes that can be placed in the wedge (Figure 5). This system is an obvious model for stability studies, but handling (freezing, thawing) procedures have to be carefully designed, and personnel need proper training. More commonly, small cylindrical stainless steel tanks are used for stability studies, although some correlation with large-scale data can be useful (9). When using improvised tanks, consideration should be given to materials of construction and surface finish. Large-scale commercial tanks and vessels are generally electropolished whereas small-scale tanks have a chemically passivated grit finish, although the latter can also be electropolished. Surface roughness can influence ice nucleation and metal leaching rates.
The S3 scaled-down system for disposable bag freezing uses small bags with linear heat transfer dimensions that are the same as for full-scale bags in the Celsius system. The materials of construction (and contact materials) are the same, although the films are slightly less thick in S3 bags. A full-scale process can therefore be simulated in small bags (30–100 mL). This system is well suited for process development and can be readily used for stability studies (10,11).
Shamlou et al. designed a bulk freezing container (≤2-L size) using rectangular geometry so the heat transfer path remains the same between a small-scale device for process development (30-mL volume) and the larger container (12). This allows for linear scaling, which is not possible using cylindrical geometries. However, linear scaling would be impractical for very large volumes.
A Focused Approach
Storage of frozen bulk protein solution is a necessity for process economics. This operation is generally developed empirically because most literature studies on protein freezing have been performed on miniature scale. The fundamental aspects important for developing robust formulations and processes are reviewed here along with available technical options to guide practitioners. The general effects of freezing on biologics are known, but practical solutions to prevent long-term storage issues are limited.
Empirically designed systems are available for process control during freezing and thawing. However, ice nucleation and growth are not controlled, although they ultimately determine long-term frozen-storage behavior. So formulations must be designed to enable a biologic to be stored frozen as well as to provide adequate stability once it has been converted to a drug product.
Stability in the frozen state cannot be assessed simply by performing FT cycling experiments. Formulation development strategies should also include manipulation of
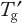
and long-term storage studies at different freezer conditions. Better understanding of practical systems is needed to determine the impact of process and solution conditions on process outcomes. Bringing chemical engineering and biophysics into play with computational fluid dynamics models for mass and heat transfer, would be useful for that purpose.
REFERENCES
1.) Singh, SK. 2009. Large-Scale Freezing of Biologics: A Practitioner’s Review, Part 1 — Freezing Mechanisms. BioProcess Int. 7:32-44.
2.) Singh, SK. 2009. Best Practices for Formulation and Manufacturing of Biotech Drug Products. BioPharm Int. 22:32-48.
3.) Schmidt, R. 2008.. Freeze and Thaw of Monoclonal Antibody Solution.
4.) Wisniewski, R, and V. Wu Avis, KE and VL. 1996.Large-Scale Freezing and Thawing of Biopharmaceutical ProductsBiotechnology and Biopharmaceutical Manufacturing, Processing, and Preservation, Interpharm Press, Buffalo Grove:7-59.
5.) Webb, SD. 2002. Freezing Biopharmaceuticals Using Common Techniques — and the Magnitude of Bulk-Scale Freeze-Concentration. BioPharm 15:22-34.
6.) Wisniewski, R. 1998. Large-Scale Cryopreservation of Cells, Cell Components, and Biological Solutions. BioPharm 11:42-50.
7.) Wisniewski, R. 1998. Developing Large-Scale Cryopreservation Systems for Biopharmaceutical Products. BioPharm 11:50-56.
8.) Kolhe, P. 2009. Large-Scale Freezing of Biologics: Understanding Protein and Solute Concentration Changes in a Cryovessel. BioProcess Int. submitted.
9.) 2009. Avastin EPAR Scientific Discussions, European Medicines Agency, London.
10.) Lashmar, UT, M Vanderburgh, and SJ. Little. 2007. Bulk Freeze-Thawing of Macromolecules: Effect of Cryoconcentration on Their Formulation and Stability. BioProcess Int. 5:44-54.
11.) Ho, K. 2008. Development of Freeze and Thaw Processses for Bulk Biologics in Disposable Bags. Am. Pharmaceut. Rev. 11:64-70.
12.) Shamlou, PA.. 2007. A New Scaleable Freeze-Thaw Technology for Bulk Protein Solutions. Biotechnol. Appl. Biochem. 46:13-26.