
Figure 1: Basic principles of technology transfer
In the biopharmaceutical industry, technology transfer refers to transfer of any process, together with its documentation and professional expertise, between development and manufacture or between manufacturing sites (1). This operation is common in the biopharmaceutical industry for a number of structural reasons. They include the dichotomy between small, innovation-based drug companies and large ones able to conduct late-phase clinical development and endowed with manufacturing capacity; the high capital cost of biopharmaceutical plants, which makes contract manufacturing attractive; and the need for multiple scale-ups during a product’s life cycle.
“Tech transfer” is a delicate operation carrying business, regulatory, product quality, and technical risks. The basic principle is simple enough: Originators transfer all their know-how to recipients to enable product manufacture. Even in that basic rendering, some difficulties are detectable: It is not a physical asset that is being relocated or duplicated, but an ability — something much harder to define, specify, and get right. Both originators and recipients might need to overcome geographical, organizational, technical, and other discontinuities. Add to those concerns the delicate nature of biopharmaceutical processing, and the complexities of tech transfer emerge: It is a difficult-to-define task relating to inherently complex manufacturing processes that can have multiple variations depending on what discontinuities must be addressed.
Each tech transfer case is potentially different and requires a distinct approach — or at least a tailored one. Below, I categorize what makes one tech transfer different from another and therefore potentially more or less complex. Such determinants of complexity have specific consequences for tech transfer projects, their critical success factors, and their risk management.
Determinants of Complexity
Many things make a given technology transfer project unique. They can be identified from the diverse situations encountered and the specific discontinuities that occur between donor and recipient sites. Table 1 lists differentiating factors along with example cases and specific risk factors. Each factor is explained briefly below, and some factors receive more detailed discussion in subsequent sections.
 An obvious determinant of complexity is how much of a production chain is transferred: from a single element (e.g., drug product fill and finish) to an entire process (from cell-bank manufacture to final drug product, with all the associated testing). Transfer scope is, up to a point, a management decision with operational and strategic elements. The larger the scope, the more complex the transfer. Complexity increases even more if different sections (e.g., drug substance, drug product, and analytics) are transferred from or to different sites.
An obvious determinant of complexity is how much of a production chain is transferred: from a single element (e.g., drug product fill and finish) to an entire process (from cell-bank manufacture to final drug product, with all the associated testing). Transfer scope is, up to a point, a management decision with operational and strategic elements. The larger the scope, the more complex the transfer. Complexity increases even more if different sections (e.g., drug substance, drug product, and analytics) are transferred from or to different sites.
Certain processes have more inherent complexity than others do. Although we automatically think of the process itself, including nonrobust steps and unusual unit operations, other causes should not be forgotten. Difficulties may stem from a molecule’s being fragile or difficult to analyze, tight specifications, raw materials being difficult to qualify, or a complex analytical package. The latter is worth special mention because nonrobustness can appear during tech transfer even for standard methods such as host-cell protein detection by enzyme-linked immunosorbent assay (ELISA). And many potency methods, especially cell-based assays, are problematic to transfer because of their high variability. Transferring an analytical package for a cell therapy is not trivial, for instance. The ability to handle innate manufacturing complexity in all its dimensions is a critical factor for an originator’s selection of a receiving site.
Changes during tech transfer are tempting and common, but they require careful management from quality, regulatory, and technical perspectives. If nothing changed, tech transfer would be reduced to a training exercise, so the type and extent of changes are clear determinants of complexity. Dealing with changes is discussed below. It involves the same approach as does innate complexity, so discussion of one can cover for the other.
Product life-cycle stage is an important differentiator among tech transfer projects. Two opposing trends are linked to it. On one hand, the farther a product moves through its clinical program, the higher the regulatory expectations will be during transfer, increasing project difficulty. On the other hand, more process knowledge is gained, which in theory should facilitate transfer.
Maturity of process technology is linked largely to a project’s clinical phase (through technical development and manufacturing experience). But even at a given clinical phase, originator understanding differs significantly among projects. Assay qualification and validation also are far from standard across projects. Such factors interact with inherent process complexity. Transferring a complex process is even riskier than normal if it is somewhat underdeveloped for its clinical phase.
A receiving site’s maturity can be measured by its experience with similar transfers, the extent and depth of its quality system, its regulatory experience, and the strength of its support functions (e.g., for development, production support, analytical, project management, and validation). Receiving-site maturity should correspond with the transfer to be executed. Nonetheless, a balance with the originator site should be maintained.
Such considerations lead to comparing the cultures of originator and receiving sites and identifying cultural difference as a source of complexity. Far more than geographic or linguistic differences is at stake, although such differences create obvious challenges.
Finally, contractual arrangements can influence technology transfer. Usually a transfer project is contracted as a work package. Although tight budgets and timelines can lead to efficient and creative solutions, they tend to place undue stress on transfer teams, damage the long-term originator–recipient relationship, and finish overspent. A quality agreement should be put in place as early as possible during transfer. Handling changes and deviations is a part of every transfer, and a joint approach must be defined. Finally, tech transfer is a defined project but rarely an end in itself: Its success criteria should be framed by a longer-term manufacturing agreement.
Technology Transfer and Product Life Cycle
Need for technology transfer can occur over a product’s entire life cycle, from the first transfer from development into good manufacturing practice (GMP) production until end-of-life transitions into low–cost-base facilities. It is key to understand that tech transfer always carries significant regulatory implications.
Initial transfer into GMP corresponds to investigational new drug (IND) and/or investigational medicinal product dossier (IMPD) submission, setting the tone for the rest of a project. After that, regulatory agencies consider every technology transfer to be a major change (apart from a few special cases). Unlike with chemical entities, there is no regulatory tolerance to a “within 10-fold scale change” for biopharmaceuticals. Even with no obvious changes, authorities are wary of new manufacturing sites because they usually have different quality management systems. Transfer always comes with a number of “hidden” changes, and often requires training staff on a new process.
The chief concern is product comparability. Regulators expect that a product made at a receiver site will be comparable clinically to a product made at the corresponding originator’s site — and that such comparability be demonstrated (2).
Several implications are worth highlighting here. First, establishing comparability is an integral part of tech transfer. Second, the burden of comparability increases with clinical experience. Relatively minor when going from development to GMP, comparability becomes exacting for commercial stages, in which large manufacturing data sets might be required.
Third, meeting specifications is usually insufficient to establish comparability. For instance, if a specification is broad compared with assay precision, data from both sites might be statistically different while still falling inside the specification. That concern might or might not have clinical implications, but an originator must address the difference. Comparability also requires more than release assays, with additional characterization and stability data (preferably accelerated or stressed).
Fourth, if analytical methods are transferred, then it is mandatory to demonstrate that they yield equivalent results. That requirement must be incorporated in analytical method transfer, and the sooner that is done in an overall program, the better.
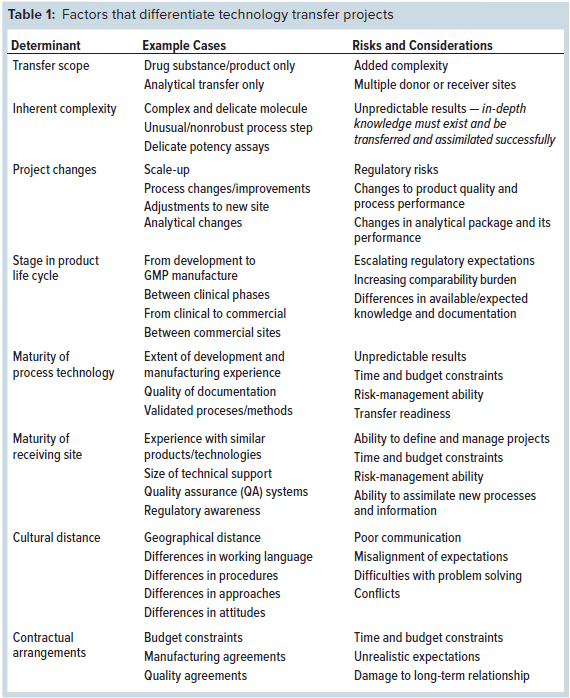
The requirement to demonstrate comparability makes tech transfer better performed at certain points than at others. Figure 2 shows life-cycle phases and major development milestones.
Figure 2: Technology transfer possibilities within a product life cycle, from preclinical studies to commercialization; optimal transfer points are indicated, as are the latest times for analytical validation (AV) and process-performance qualification (PPQ).
Some basic recommendations can be given. Authorities will not welcome a major manufacturing change within a clinical phase because it could be a confounding factor, and such a change does not reflect smart project planning. It is best to revise a chemistry, manufacturing, and controls (CMC) section at clinical transition points.
Tech transfer just before commercial registration also is highly undesirable. If all pivotal clinical data have been generated using material made at site A, authorities will hesitate to approve a commercial launch with material made at site B because no clinical coverage exists for that new material. Ideally, the same site should be used for final clinical and early commercial material. If additional capacity is needed, a commercial-to-commercial transfer is best.
Such regulatory preferences, which are not always well understood, can cause problems — e.g., with new sites designed primarily for commercial manufacture. Figure 2 shows an optimal series of transfer transitions.
As demonstrated earlier in this paper, comparability burden and transfer complexity depend significantly on life-cycle position. But so do other expectations, especially everything related to validation (Figure 2). Before a process is validated, that activity is not usually included in a transfer scope. But after validation, revalidation must be included. Analytical methods may be transferred by comparison testing/covalidation if they are not already validated — or by revalidation if they are. Consequently, work packages and documents to be produced as tech transfer deliverables differ significantly over a product’s life cycle.
Life-cycle position strongly influences the nature and amount of information to transfer — and thus, transfer complexity. Early on, there is little manufacturing experience or process characterization; analytical methods might be incomplete and barely qualified. In commercial phases, methods and processes are validated, knowledge from extensive characterization is available, and a receiving site can benefit from a large manufacturing history, including trending data and experience with diverse changes and significant deviations. If such knowledge can be communicated efficiently and successfully, then tech transfer should be facilitated and derisked considerably.
Changes During Tech Transfer
Making changes during technology transfer is tempting and common, but their implications require careful management — and not only for regulatory reasons.
Scale-up: Scale-up is common, especially in early clinical phases. At first glance, it might not be considered a change because most of the associated processes can be described in scale-independent ways. Yet practice and theory show that many factors can change during scale-up. A proper discussion of them is beyond the scope of my article. Suffice it to say that difficulties tend to come from hydrodynamics and flow regimes that can change dramatically even within an order-of-magnitude scale-up. That alters mass transfer, mixing, and sometimes shear.
The good news is that scale-up difficulties readily can be approached through progressive scale increases, ending with an engineering run. However, such concerns are hard to identify from small-scale experiments alone, and remedying problems is tough. Significant changes in equipment design can be put in the same category as scale-up.
Changes: With some overlap, changes come in three types that are applicable to upstream and downstream processes as well as analytical methods: deliberate, induced, and hidden changes.
Deliberate changes are those decided independently from receiver-site selection: e.g., introduction of a working cell bank, addition or significant optimization of a purification step, and addition or replacement of an analytical method.
Induced changes are substantive modifications created by a tech transfer itself. They greatly depend on receiver-site selection (which induces them). Such adaptations include using a depth or microfilter instead of a centrifuge for primary separation, introducing multiple cycles on a chromatography step or changing a column’s diameter, and implementing a freeze–thaw step of an intermediate to accommodate site organization. Some equipment-related changes to process parameters fall into this category. Induced changes can be identified at any point in a transfer program, though a diligent team will identify them early.
Hidden changes are usually small and come to light only through detailed comparison of a process at both sites. Some changes never come to light or do so only by chance. Consider variations in raw material properties, changes linked to equipment design (vessel aspect ratios and pump and impeller types), differences in timing (for heating/cooling, mixing, and holding), and variations in media and buffer preparation details.
Deliberate and induced changes almost always require regulatory notification and should be treated with specific change-control actions. That is not the case for hidden changes, which usually can be managed under a “blanket” approach. The difference between deliberate and induced changes is that an originator chooses the former, whereas the latter are consequences of selecting a receiving site. Hidden changes also depend on conditions at a receiving site, but it is impossible to eliminate them all.
Both deliberate and induced changes are relatively amenable to a structured experimental approach and risk management. Consequences of these changes are quite similar to those that accompany intrinsically difficult processes: They require more elaborate programs, place a premium on experience and knowledge at both sites, and require superior knowledge transfer.
Hidden changes can be difficult to handle. If nothing is done, they will remain hidden until it might be too late, but too much emphasis on tracing them can waste precious resources. Yet we all have experienced some small process change that created an undesired effect.
Risk identification and evaluation are recommended. Hidden changes can be identified through brainstorming, using checklists, going through parameter lists, using fishbone diagrams or the 6M method, and performing process walk-downs. Process walk-downs (or facility-fit analyses) consist of a team visualizing, in as much detail as possible, a process as it would be carried out in a new facility and systematically identifying differences between facilities. Once identified, changes can be assessed for possible impact with typical risk-analysis tools, and actions can be taken. These tend to be mitigative rather than corrective. Often, hidden differences are linked to receiver-site operations and are, therefore, difficult to reverse.
Dealing with changes of whatever type takes time and requires a joint team comprising members from both sites. At first, this can seem to be a constraint, but conducting thoughtful analyses and defining approaches can be powerful means of team building between sites. A joint team also provides a forum for process information to surface and be shared.
Figure 3: Technology transfer must bring two cultures together because it occurs over a discontinuity in which the human factor can be significant.
Cultural Distance Between Donor and Recipient Sites
Figure 3 illustrates hurdles presented by cultural differences between two sites. Originator and recipient sites usually represent different organizations, and they can be geographically remote, often in countries that do not share a language. It is likely that their corporate and social cultures will differ. That includes different procedures, habits, behaviors, expectations and norms, and even ways of thinking. During tech transfer, two different mindsets must be brought together, then made to adjust and communicate satisfactorily. Unity is both a precondition and an achievement of a successful tech transfer.
Stating that communication is critically important to tech transfer is a truism, but it is still worth exploring why. Apart from a few material elements (such as cell banks and reference standards), the only thing that actually is transferred is information. That depends entirely on communication. Tech transfer complexity also is a factor, so timing, accuracy, and completeness of information (whether emitted or received) are all essential. Information comes in many forms, from the relatively easy to communicate (e.g., buffer composition) to the much more difficult (what controls a critical quality attribute of a molecule).
The nature and format of information shared during technology transfer could be the subject of a separate article. Nonetheless, much tech transfer information is latent rather than apparent, partly because providing too many sources (e.g., all development reports) can be self-defeating and partly because know-how surfaces only when the right questions are asked.
Geographical distance, which is better measured in travel time or cost than in miles, is an obvious obstacle that information technology (IT) can help to negotiate. However, there is no substitute for face-to-face contact, particularly when cultural distance is significant. Investing in face-to-face meetings during technology transfer pays dividends not only for a project, but also for a collaboration.
Language barriers should not be underestimated. Even if everyone spoke “airport English,” that would remain a shallow means of expression prone to all sorts of misunderstandings. Fluent speakers may not realize that they are not being understood. And those less able might be shy to say that they are not understanding. In such situations, humor can be misinterpreted, and subtleties can be lost.
Cultural traits are important because they condition reactions, emotions, and ultimately engagement. These traits will emerge at times of tension and conflict, which are inevitable during tech transfer because different interests are being managed. On a more rational level, corporate differences mean that approaches and procedures can differ. Originator and recipient both will have deviation procedures, but they might categorize deviations differently. Similar statements hold for all procedures, and the cumulative effect can be quite large.
Clearly, the greater the cultural difference, the more challenging the tech transfer. But there are ways to address differences. Structuring communication is a good start. The importance of face-to-face meetings has been mentioned, and they can take different forms: project and business meetings, presentations, workshops, and even team-building events. Working together is critically important, either through one-on-one interactions between experts or as teams. In-person interactions build trust, relationships, and understanding; enable teams to learn each other’s procedures; and promote information flow. During such interactions, important details that cannot be gleaned from structured documents get flushed out. All of this requires time, but that time is well spent and can be made productive — e.g., by doing joint risk assessments.
Analyzing Complexity to Improve Tech Transfer
I have identified several sources of complexity and analyzed three in detail: stage of product life cycle, project changes, and cultural distance. But how can recognizing complexity improve tech transfer? First, two points must be made about complexity criteria. For one, they are not usually independent. The requisite level of technology maturity depends on a drug’s life-cycle position, for instance. Nevertheless, analyzing a transfer project in such terms helps identify important and risk-prone elements.
The second point is that the complexity criteria exacerbate one another during tech transfer, so their cumultaive effects tend to be worse than merely additive. Imagine transferring a complex process with a couple of delicate critical assays, knowing that drastic development shortcuts have been taken, to a site quite unused to your technology and to a team that functions with a different mindset.
Thinking of tech transfer along different dimensions of complexity is helpful in several ways. Doing so can generate clear criteria for recipient-site (or contract manufacturer) selection. Originators should not limit their inquiries to process fit and batch cost. Certain sites are better for early phase projects, whereas others excel at later phases.
Cultural fit — or the ability to work together — may be the most important criterion of all. Although difficult to evaluate up front, it is fortunately amenable to improvement.
Analyzing complexity helps formulate a transfer project. Project elements will depend heavily on transfer scope and technological complexity, the number of changes, a product’s life-cycle position, and the extent of information to be delivered. Risks inherent to a project will depend on those dimensions as well as on the others explored above (e.g., maturity of originator/recipient and cultural distance).
Complexity criteria help delineate two main components of a transfer program. The information package is one aspect: its content, format, and communication vectors. The other is the experimental program prior to GMP manufacturing, the scope of which can range from laboratory experiments to engineering runs at full scale.
Because it identifies important transfer characteristics, complexity analysis allows a project to be defined and tailored to a particular case. I have given some illustrative elements of this above: e.g., adjusting a program based on life-cycle position, identifying and managing changes, and fostering productive interactions. The ways in which complexity criteria can shape an information package and experimental program warrant further attention.
References
1 WHO Guidelines on Transfer of Technology in Pharmaceutical Manufacturing. World Health Organization: Geneva, Switzerland, 2011; https://www.who.int/medicines/areas/quality_safety/quality_assurance.
2 ICH Q5E: Comparability of Biotechnological/Biological Products Subject to Changes in Their Manufacturing Process. International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use: Geneva, Switzerland, 2004; https://database.ich.org/sites/default/files/Q5E%20Guideline.pdf.
Thomas Chattaway ([email protected]) has over 30 years of experience in the life sciences, with a focus on biotechnology, process/product development, technology transfer and validation, contract manufacturing, and quality assurance. As a consultant, he mainly pursues CMC topics but also has a keen interest in access to medicines for low- and middle-income countries.











