Tissue engineering is a multidisciplinary science that applies principles from engineering to the biological sciences to create replacement tissues from their cellular components (1). Resulting neotissues can repair or replace native tissues that are diseased, damaged, or congenitally absent. One technique that has come into widespread use is based on seeding cells onto a three-dimensional (3D) biodegradable scaffold that functions as a cell-delivery vehicle (2). Cells attach to the scaffold, which then provides space for neotissue formation and can serve as a template for directing neotissue development. As a scaffold degrades, new tissue forms — ultimately creating a purely biological substrate without any synthetic components.
Cells seeded onto a scaffold can be harvested from one patient who requires a tissue-engineered construct, thus eliminating the risk of immune rejection. The resulting fully autologous neotissue is perfectly biocompatible and can become completely integrated into the host. Neotissue is a living construct that can use a patient’s innate biological mechanisms for growth, repair, and remodeling. As such, it has excellent durability and is uniquely suited for use in children, for whom currently used synthetic biomaterials lack growth capacity and are subject to somatic overgrowth (3). With this scaffolding process, a growing child can obviate the need for a second “redo” surgical procedure that could be fraught with complications and is usually required to replace synthetic vascular grafts that children outgrow.
Perhaps nowhere in medicine is the promise of tissue engineering greater than in the field of congenital heart surgery. Taken together, congenital cardiac anomalies are the most common birth defects in humans, affecting nearly 1% of all live births (4). Despite significant advances in both the surgical and medical management of such patients, congenital heart disease remains a leading cause of death in newborns (3). One significant source of morbidity and mortality comes from complications that arise from the use of synthetic biomaterials in the form of vascular grafts, patches, or replacement heart valves for congenital heart operations.
The main concern in such procedures is acquisition of suitable “conduits” or structures that can be used to function in vivo to create new blood vessels. Devices pose a significant source of complications, including thromboembolic events and infectious contamination. In addition, they have poor durability due to ectopic calcification, neointimal hyperplasia, and somatic overgrowth. Additional complications include bleeding, aneurysmal dilation, and rupture. There is a small cohort of patients who, due to the nature of their anatomic defects, can undergo reconstructive cardiac surgery without needing any synthetic biomaterials and thus eliminate complications associated with their use (5). That cohort of patients fares particularly well and has been the impetus for our research.
We developed the first tissue-engineered vascular graft (TEVG) to be used in humans (6). Our TEVG is created by seeding autologous, bone-marrow–derived mononuclear cells (BM-MNC) onto a biodegradable scaffold fabricated from polyglycolic acid fibers that are knitted into a tube and coated with a 50:50 copolymer of L-lactide-∊-caprolactone (P(LA/CL)) from Gunze Ltd. (Photo 1). A seeded construct is bathed in autologous plasma (also collected from bone marrow) for two hours before its implantation as a vascular conduit. We have demonstrated the feasibility of using TEVGs in surgical repair of congenital cardiac anomalies in children (7). We targeted the pediatric patient population to take advantage of unique features of TEVGs — in particular, their growth potential (8). We are currently performing the first FDA-approved clinical investigation to evaluate the safety and growth potential of TEVGs, having performed the first US implantation in a child over a year ago, in August 2011. That patient is doing well at this time.
Photo 1:
Photo 1: ()
Seeding the Constructs
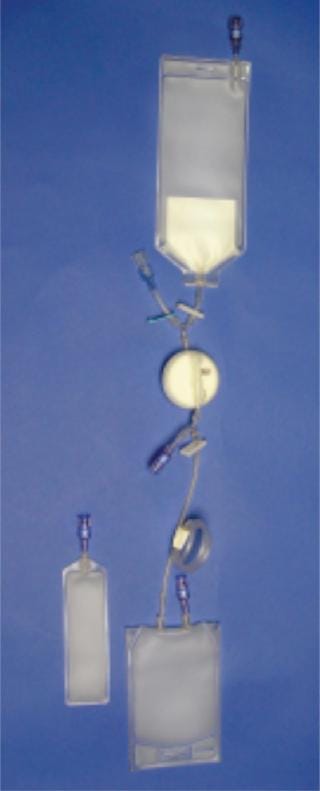
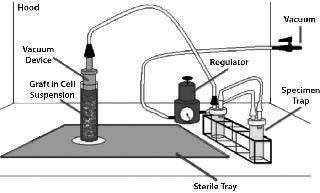
One significant factor limiting the clinical utility of TEVGs is the fact that it requires an ISO class 7 or class 10,000 room for isolating BM-MNCs, seeding cells onto a scaffold, and incubating the seeded construct before implantation. Use of such facilities is both labor-intensive and costly. To eliminate that problem (and in collaboration with Pall Corporation and Gunze Ltd.), we developed a prototype for a closed, disposable, filter–vacuum seeding system for isolating autologous BM-MNCs (Figure 1), seeding them onto a tubular biodegradable scaffold, and then incubating the seeded construct (9). The basis for creating such a closed, disposable system is founded on changing our cell-isolation method from Ficoll density centrifugation to trapping BM-MNCs using a leukoharvest filter from Pall (Photo 2) and eluting mononuclear cells off that filter with an elution solution (10).
Photo 2:
Photo 2: ()
Figure 1: ()
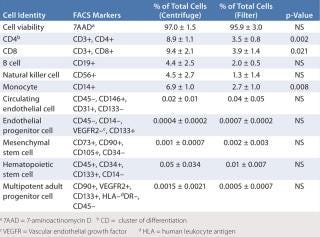
As an initial test to evaluate the feasibility of using this new method for creating a TEVG, we compared preliminary characterization of cells isolated from bone marrow using Ficoll density centrifugation with those isolated using a leukoharvest filter from Pall Corporation (Table 1). Next, we compared the patency of TEVGs created using BM-MNCs isolated with both techniques. Our results demonstrated some minor differences in the subpopulations of mononuclear cells, but showed no difference in their ability to promote formation of vascular neo
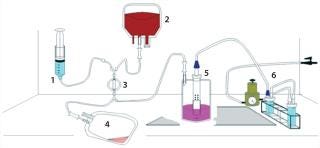
tissue as demonstrated by graft patency (10). We subsequently designed a closed, disposable, filtration–vacuum seeding system using the Pall leukoreduction filter, the Gunze scaffold, and our vacuum-seeding method, then built a prototype (Figure 2).
Figure 2: 1) elution solution, (2) bone marrow container, (3) filter, (4) plasma collection bag, (5) seeding chamber (with scaffold loaded onto mandril), and (6 ()
Table 1: Comparative fluorescence-activated cell-sorting (FACS) analysis of bone-marrow–derived mononuclear cells isolated using Ficoll density centrifugation and Pall filter entrapment/elution (adapted from 10)
Table 1: Comparative fluorescence-activated cell-sorting (FACS) analysis of bone-marrow–derived mononuclear cells isolated using Ficoll density centrifugation and Pall filter entrapment/elution (adapted from 10) ()
Putting It into Practice
We have begun a large animal study evaluating the use of our closed, disposable, filtration–vacuum seeding system to create a TEVG. The TEVGs are being implanted as cell-seeded intrathoracic inferior vena cava (IVC) interposition grafts in juvenile lambs. Results compared with those seen using both unseeded scaffolds and those seeded with BM-MNCs isolated using Ficoll density centrifugation.
We developed and validated this model system to evaluate TEVGs for use in congenital heart surgery. We have also used the same model system to evaluate the growth potential of TEVG in vivo (11). And we are continuing to characterize the closed, disposable, filtration–vacuum seeding system to enable it to eliminate the need for an ISO class 7 cleanroom in our FDA-approved clinical investigation that evaluates using TEVG in congenital heart surgery.
Identifying biomarkers for monitoring the development of vascular neotissue would facilitate translation of this technology to the clinic (12). We recently published our findings that cells seeded onto a TEVG are rapidly lost from the seeded scaffold after implantation (13). Next, we demonstrated that the TEVG forms a neovessel that resembles a native blood vessel by inducing migration of endothelial cells and smooth-muscle cells from the neighboring blood vessel wall to generate a blood vessel (14). More recently, we proved that neovessel formation requires infiltration of host-derived macrophages into the scaffold wall — and that excessive infiltration of host macrophages leads to development of TEVG stenosis (15). Thus, quantification of the degree of macrophage infiltration provides us with a biomarker for evaluating neotissue development during neovessel formation.
Our studies have provided proof of principle for the utility of a closed, disposable system that can be used for point-of-care treatment and thus eliminate the cost and complexities associated with building, using, and maintaining an ISO class 7 facility. The closed, disposable, filtration–vacuum seeding system could provide a platform technology that is well suited for any tissue-engineering application using autologous, bone-marrow–derived mononuclear cells (or any subset of that diverse cell population). Bone-marrow–derived mononuclear cells have been used for a number of other applications besides vascular grafts.
Current investigations are under way to develop and translate products as diverse as replacement heart valves and replacement tracheas. Thus, we envision several applications for this sort of cell-harvesting system. As more tissue-engineering applications begin the process of translation from the laboratory bench to clinical application, the development of enabling technologies such as this closed, disposable, filtration–vacuum seeding system will be critical to the safe and efficacious use of these new therapeutic procedures.
About the Author
Author Details
Corresponding author Christopher Breuer, MD, and Toshiharu Shinoka, MD/PhD, are codirectors of the tissue-engineering program at Nationwide Children’s Hospital, 700 Childrens Drive, Columbus, OH 43205; 1-614-722-2000; [email protected]. Edward Snyder, MD, is a professor in the department of laboratory medicine at Yale University’s School of Medicine in New Haven, CT.
REFERENCES
1.) Langer, R, and JP Vacanti. 1993. Tissue Engineering. Science 260:920-926.
2.) Khademhosseini, A, JP Vacanti, and R Langer. 2009. Progress in Tissue Engineering. Sci. Amer. 300:64-71.
3.) Patterson, JT. 2012. Tissue-Engineered Vascular Grafts for Use in the Treatment of Congenital Heart Disease: From the Bench to the Bedside and Back Again. Regen. Med. 7:409-419.
4.) Hoffman, JIE, and S Kaplan. 2002. The Incidence of Congenital Heart Disease. JACC 39:1890-1900.
5.) Van Son, JA, M Reddy, and FL Hanley. 1995. Extracardic Modifications of the Fontan Operation Without Use of Prosthetic Material. J. Thorac. Cardiovasc. Surg. 110:1766-1768.
6.) Shinoka, T, Y Imai, and Y Ikada. 2001. Transplantation of a Tissue Engineered Pulmonary Artery. NEJM 344:532-533.
7.) Shinoka, T. 2005. Midterm Clinical Result of Tissue Engineered Vascular Autografts Seeded with Autologous Bone Marrow Cells. J. Thorac. Cardiovasc. Surg. 129:1330-1338.
8.) Hibino, N. 2010. Late-Term Results of Tissue-Engineered Vascular Grafts in Humans. J. Thorac. Cardiovasc. Surg. 139:431-436.
9.) Udelsman, B. 2011. Development of an Operator-Independent Method for Seeding Tissue-Engineered Vascular Grafts. Tiss. Eng. Part C Meth. 17:731-736.
10.) Hibino, N. 2011. Comparison of Human Bone Marrow Mononuclear Cell Isolation Methods for Creating Tissue-Engineered Vascular Grafts: Novel Filter System Versus Density Centrifugation Method. Tiss. Eng. Part C Meth. 17:993-998.
11.) Brennan, MP. 2008. Tissue-Engineered Vascular Grafts Demonstrate Evidence of Growth and Development When Implanted in a Juvenile Animal Model. Ann. Surg. 248:370-377.
12.) Prestwich, GD. 2012. What Is
the Greatest Regulatory Challenge in the Translation of Biomaterials to the Clinic?. Sci. Transl. Med. 1:14.
13.) Roh, JD. 2010. Tissue-Engineered Vascular Grafts Transform into Mature Blood Vessels Via an Inflammation-Mediated Process of Vascular Remodeling. Proc. Nat. Acad. Sci. USA 107:4669-4674.
14.) Hibino, N. 2011. A Critical Role for Macrophages in Neovessel Formation and the Development of Stenosis in Tissue-Engineered Vascular Grafts. FASEB J. 25:4253-4263.
15.) Hibino, N. 2011. Tissue-Engineered Vascular Grafts Form Neovessels That Arise from Regeneration of the Adjacent Blood Vessel. FASEB J. 25:2731-2739.