Vaccines are powerful and cost effective prophylactic tools for protecting public health. The Global Alliance for Vaccines and Immunizations (GAVI) estimates that ~5.4 million lives are saved each year by the administration of vaccines for hepatitis B, measles, haemophilus influenza type B (hib), pertussis (whooping cough), yellow fever, and polio (1). According to the World Health Organization, seasonal influenza alone claims 250,000–500,000 lives every year globally, many of which could be prevented by more widespread vaccination with the seasonal influenza vaccine (2). Currently, vaccines are available for 26 indications, and those for at least 12 more indications are in clinical trials, with anticipated approval and launch in the next five years (3). Overall, the worldwide vaccine market registered revenues of US$10.6 billion in 2005 and $21 billion in 2008, and they are projected to increase to $35 billion by 2015 (4, 5). Thus vaccines represent a clinically important sector as well as one of the fastest commercial growth sectors within the biopharmaceuticals market.
PPRODUCT FOCUS: Vaccines (and protein biologics)
PROCESS FOCUS: Downstream processing; formulation, fill–finish
WHO SHOULD READ: QA/QC, PROCESS DEVELOPMENT, AND MANUFACTURING
KEYWORDS: BULK FREEZING, STABILITY, DISPOSABLES, THERMAL CONTROL, AND CRYOCONCENTRATION
LEVEL: INTERMEDIATE
Vaccines typically contain one or more active ingredients that are used to stimulate an immunogenic response upon administration to a patient. Maintaining the stability and (in the case of live attenuated vaccines) viability of the active ingredients throughout production, distribution, and administration is a major challenge in vaccine manufacture.
This challenge stems from the active ingredients’ marginal stability. Unformulated active ingredients are often stable for only a few hours at room temperature and a few days or weeks under refrigerated conditions (2–8 °C). Vaccine manufacturers implement a number of strategies are to minimize their active ingredients’ instability-related product losses throughout the product life-cycle.
Within a production environment, two main strategies are available: continuous production to finished product within a preestablished time limit or discontinuous production in which product purification activities are decoupled from subsequent formulation, fill, and finish activities.
That decoupled production strategy (where feasible) is preferred by most manufacturers because it provides flexibility in production schedules as well as in the physical locations of fill– finish facilities. This is especially relevant for manufacturing multivalent vaccines in contract manufacturing settings. The critical link for decoupled production steps is an ability to freeze and later thaw bulk drug substance (DS) without loss of activity. Here we briefly review several commonly used freezing options for vaccine manufacture, then focus on the use of blast freezers for rapid freezing drug substances in disposable containers.
Why Is Rapid Freezing Necessary?
The underlying rationale for freezing DS is to eliminate or at least slow down the rate of active-ingredient (AI) degradation by storing products at temperatures below the glass transition temperature of the AI (6). However, depending on the degree of the freeze-induced stress on the DS, the severity of negative consequences caused by freezing may vary. For some vaccine products — such as live attenuated virus vaccines — the rate at which the DS is frozen (degrees reduced per hour) can be important in maintaining viability of the viruses (which constitute the AI). It is not uncommon to lose 50% of the AI during a freeze–thaw cycle and its subsequent filtration step (7).
Photo 1 shows the difference in visual appearance between two thawed samples of the same lot of a live influenza virus harvest fluid. Samples were frozen at very fast or very slow freezing rates, then thawed to room temperature at the same rate. The fast-freeze sample was frozen by immersion in liquid nitrogen (freezing rate of ≥–300 °C/h), whereas the slow freeze sample was frozen at –1 °C/h. Both samples were thawed in a 25 °C water bath with mixing by inversion at regular intervals. Similar experiments were performed using different inf luenza strains, for which viral potency losses in the slow-freeze samples ranged 19–56% depending on the virus strain. This contrasts with no observed loss in viral potency of the fast-freeze samples. Webb et al. (8) reported that slower freezing rates can cause high levels of cryoconcentration and damage protein structures. So faster freezing rates are preferable for reducing potency losses.
Photo 1:
Photo 1: ()
Selecting the Right Freezing Equipment
In a typical vaccine manufacturing setting where DS freezing is performed, the active ingredient is concentrated using ultrafiltration or isopycnic ultracentrifugation to reduce the storage volume and remove excess water. At minimum, a cryoprotectant such as sucrose is added to stabilize the AI during freezing and thawing (7). This is sometimes referred to as preformulation. Because extremely fast freezing rates ≥–300 °C/h are not necessarily feasible in a production environment, process engineers have to experimentally determine the slowest acceptable freezing rate that will minimize product losses in a preformulated DS.
In some cases, choosing the right preformulation excipients and minimal freezing rate may be an iterative process until the best combination is found. The scale and the minimum freezing rate provide the basis for selecting appropriate freezing equipment and also for determining load configuration per batch for validation purposes. With products such as live attenuated virus vaccines — for which high-dose yields per batch are common — medium-scale volumes of 4–8 L of concentrated DS can yield millions of doses. Typically, the concentrated DS generated is only a few liters in volume per batch.
Inadequacy of Conventional Freezers: Product losses are expected when DS freezing is carried out using conventional upright or chest freezers with set-point temperatures ranging from –20 °C to –80 °C. This can be attributed to the inadequacy of such freezers in providing the required freezing rates for a given load configuration. In some instances, a crude solution is to use multiple freezers — “freezer farms” — and restrict the load of each one.
Conventional freezers are also challenging to validate because they lack uniform cooling throughout the entire freezer space. In some cases, “hot spots” within a freezer are identified during validation. Those are appropriately cordoned off with elaborate instructions to prevent storage of any materials in those spots. More effici
ent solutions are clearly required.
Reusable Technology — The CryoFin Stainless Steel System: An alternative option is Sartorius-Stedim Biotech’s CryoFin stainless steel system, which was specifically developed as a scalable, reusable, and validatable technology for controlled freeze–thaw and transportation of biopharmaceutical DS batches at large scale. Several articles have detailed the characteristics and performance of this equipment (8,9,10).
However, stainless steel solutions are not without drawbacks. They require high capital investment and relatively high operation and maintenance costs. Although passivation reduces the risk of metal ions leaching into products, it does not fully eliminate the problem, so the effect of trace metals on vaccine product stability needs to be considered. And stainless steel vessels often contain elastomers and gaskets that have different cooling and heating properties from the metal. If they are not replaced regularly, they can become damaged over repeated use and potentially cause leaks and sterility problems (11).
Single-use technologies offer several advantages over stainless steel reusable options (12). Dividing products among several disposable containers allows manufacturers the flexibility to thaw and use only what is needed without subjecting a whole batch to a new thaw–freeze cycle. This feature is especially relevant to seasonal vaccine manufacture, in which the shelf-life of a final product is short and demand may not be very predictable.
Despite those advantages, the disadvantages of single-use technologies must be carefully evaluated, and process development must be performed before adopting them. Leachables, extractables, and chemical compatibility data must be obtained for disposable containers just as for reusable containers, and careful risk assessment must be performed to preserve the safety and identity of a given DS.
Disposable containers lack the rigidity of traditional reusable containers. Bags need to be protected from accidental puncture and bursting. Appropriate drop tests must be performed during bag design and development to ensure integrity in the event of an accidental drop during handling. Disposable bag integrity and safety can be enhanced using appropriate support containers to provide a rigid barrier that prevents accidental puncture.
Another risk associated with disposable containers and manifolds is the reliance on specific vendors for container supply and sterilization. Disruptions in supply or sterilization can cause serious delays in vaccine and biopharmaceutical production schedules. This risk may be mitigated in part by qualifying secondary vendors.
So improved process economics, relatively few stability concerns and the “thaw-only-what-is-needed ” feature make disposables attractive. Leachables, extractables, and chemical compatibility issues are common to both reusable and disposable technologies but in different ways. And disposables are less rigid than reusables and liable to supply disruptions.
Thermal Control of Disposable Options
Single-use options for freezing essentially consist of disposable containers. They are thermally controlled either by solid-surface contact or through contact with a cold fluid such as liquid nitrogen or air.
Solid-Surface Contact–Based Disposable Technologies: An example solid-surface contact–based technology is Sartorius-Stedim Biotech’s Celsius disposable platform, which has been discussed in detail elsewhere (13). It requires use of Celsius-Pak containers from the same company to suit the bag frames and the geometry of a heat-exchanging contact plate. The lack of an alternative supplier for these disposable containers may prove to be an undesirable feature of this option. However, this is one of the most attractive options for medium to large-scale freeze–thaw applications and has been reported (13) to provide a high degree of uniformity and process reproducibility.
Cold-Fluid Contact–Based Disposable Technologies: Among cold fluids, liquid nitrogen with a boiling temperature of –196 °C is very effective in rapid product cooling and capable of exceeding the minimum freezing rate required. Such extremely cold temperatures can be detrimental, however, by causing thermal stresses that crack contact surfaces in some instances. Consequently, a number of disposable container options (such as the commonly used bags) are unusable with liquid nitrogen. In addition, it costs more than cold air. Production of liquid nitrogen requires larger spatial and carbon footprints than does production of the same volume of cold air.
Another cold-fluid option involves cold air and blast freezers to rapidly freeze the contents of disposable containers below their glass transition temperature. In a typical air-blast freezer, air cooled by mechanical compression is blown over the surface of the freezer’s contents from the top of the chamber, and warmer air is removed from the bottom of the chamber and recirculated through the mechanical air compressor. The extent of cooling achieved depends on the temperature and velocity of the air. Faster velocities and colder temperatures give faster cooling, but a decrease below the air temperature is not possible.
Although blast freezers are extensively used in the food industry and for blood-plasma storage, their use in the biopharmaceutical industry is limited thus far. To the best of our knowledge, no published literature describes the use of blast freezers in biopharmaceutical manufacturing. Here we present a case study showing freeze profiles and the extent of protein cryoconcentration in 125-mL bottles and 1-L bags using an air-blast freezer.
Study Scheme
The loads we tested were 6 L (distributed as 100 mL each in 125-mL bottles) and 8.8 L (distributed as 800 mL each in 1-L bags), respectively. We performed a conventional upright freezer process with 125-mL bottles as a control experiment. And we chose azoalbumin (Sigma) as our model protein to predict actual performance based on its ease of quantitation (well defined extinction coefficient) and for chromogenicity. High cryoconcentration levels of azoalbumin during freezing can cause a large amount of protein aggregation and consequently a potential product loss, and vice versa. Following confirmation through simulation studies that the extent of cryoconcentration is not severe, we repeated the freezing procedures with a live-atteuated virus vaccine product DS in both types of containers.
Methods and Materials
The disposable containers we used were 125-mL polycarbonate bottles from Nalgene Nunc International measuring 4 in. in height by 2 in. in width and diameter and 1-L low density polyethylene bags from Thermo Scientific HyClone measuring 10 × 7.5 × 1.4 in.
Freezers: The air-blast freezer (Thermo Electron) we used in this study had an internal chamber volume of 120 L and was equipped with a 0.5-horsepower top-mounted blower through which the cold air from a 5-horsepower compressor is blown and warm air is removed from the bottom. We set this freezer nominally to –40 °C for all studies described here.
The Harris HLT series ultralow temperature upright freezer (Thermo Scientific) we used had five shelves and a total internal chamber volume of 572 L. Each shelf provided an internal volume of 130 L. We set this freezer to –80 °C for all studies described here.
Freezing Set-Up in Blast Freezer: We used 60 125-mL bottles, each with a fill volume of 100 mL. The total liquid volume was 6 L (5% of the blast freezer’s internal chamber volume of 120 L), with all 60 bottles arranged on three shelves (20 bottles per shelf). A minimum 2-in. gap was maintained between adjacent bottles.
Separately, we used 11 1-L bags, each with a
fill volume of 800 mL. The total liquid volume was 8.8 L (~8% of the blast freezer’s internal chamber volume of 120 L). We placed each bag horizontally on its own separate shelf in the blast freezer. The shelves are uniformly separated by a 3.5-in. gap between adjacent shelves.
Freezing Set-Up in Conventional Upright Freezer: We used 60 125-mL bottles, each with a fill volume of 100 mL and all placed on one shelf. The total liquid volume was 6 L (~5% of the freezer shelf volume of 130 L). We arranged these bottles in three trays (20 bottles per tray). The bottles were evenly distributed in the shelf space, with about 1.5 in. between adjacent bottles.
Separately, we placed eight 1-L bags filled with 800 mL of liquid volume on two shelves (four bags per shelf) in the conventional upright freezer. The bags were evenly distributed, with about 3 in. between adjacent bags. We did not perform azoalbumin concentration studies with the conventional upright freezer.
Thawing Scheme: We used a Multitron II incubator shaker (ATR Biotech) for thawing with its orbital shaking speed set to 100 rpm and temperature set to 30 °C. We stopped the thawing once all containers reached refrigerated conditions (2–8 °C).
Results and Discussion
Simulation Studies to Determine Extent of Cryoconcentration: Freezing causes temperature gradients, which lead to differences in liquid density within a container. The density differences cause a convectional phenomenon in the liquid phase that is attributable to colder liquid along the walls exposed to cold temperature (14). The colder liquid along those walls freezes first, pushing and redistributing solutes toward the middle of the container. The resulting cryoconcentration patterns depend largely on the geometry of the container and direction of heat exchange.
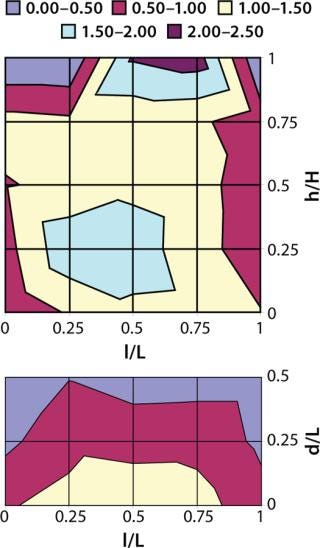
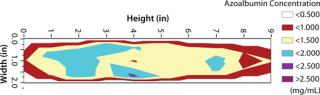
Cryoconcentration in Plastic Bottles: We froze 125-mL bottles (each f illed with 100 mL of azoalbumin in sucrose buffer solution at a concentration of 0.84 mg/mL) according to the freezing scheme described above. Then we cut the frozen containers along the vertical and horizontal planes in their centers for extensive sampling (n = 35, ≥0.1 mL each) at regularly spaced locations on the vertical and horizontal planar sections. We cut point samples from the frozen material, then melted and diluted them before testing. Protein concentrations were analyzed at a UV wavelength of 280 nm using a SpectraMax 190 microplate spectrophotometer (Molecular Devices). We plotted the resulting protein concentration data as contour plots using Microsoft Excel spreadsheets. Figures 2 and 3 show the contour plots of vertical and horizontal planar sections. (The base length is the length of the container base.) To improve the visualization and understanding of the cryoconcentration phenomenon, we froze an aqueous solution of a red under similar conditions, then cut it along the vertical planar section and photographed it (Photo 2).
That picture shows that solutes are generally depleted in the outer sections near the walls and increased in the interior sections. In particular, a two to threefold increase in azoalbumin concentrations is seen in the lower half and the top of the liquid near section near the center of the bottle. These data suggest that an upward volume expansion of the ice–liquid mixture occurs and culminates in an eruption of highly concentrated liquid near the top of the liquid surface (analogous to a volcanic eruption) (Photo 2).
Photo 2:
Photo 2: ()
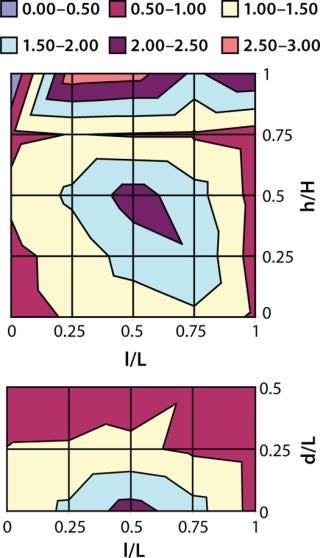
Figures 1 and 2 also illustrate a difference in cryoconcentration patterns between bottles frozen in the blast freezer and those frozen in a conventional upright freezer. The extent of cryoconcentration near the geometric center and the top center is higher in the conventional upright freezer. The highest and lowest azoalbumin concentrations were 2.27 mg/mL and 0.28 mg/mL, respectively, for the blast-freezer samples; the highest and lowest upright-freezer concentrations were 2.81 mg/mL and 0.62 mg/mL, respectively. The model protein solution used here contains only sucrose as a cryoprotectant and does not include any other stabilizing excipients. Thus, the roughly threefold variation in concentration represents a worst-case scenario for the described freezing scheme using a blast freezer.
Figure 1: ()
Figure 2: ()
Cryoconcentration in Plastic Bags: We froze 1-L bags (each filled with 800 mL of azoalbumin in sucrose buffer solution at a concentration of 0.92 mg/mL) according to the freezing scheme described above. Then we cut those frozen containers along the vertical and horizontal planes at their centers. We then melted the point samples cut from that frozen material and diluted them before testing, with extensive sampling (n = 36, ≥0.1 mL each) at regularly spaced locations on the vertical and horizontal planar sections. Protein concentrations were analyzed at 280 nm as described above. And we plotted the resulting data as contour plots using statistical software from JMP Software (Figure 3). The contour plot shows that cryoconcentration of azoalbumin in 1-L bags is similar to that in 125-mL bottles, with no evidence of intensification by the increase in volume. The highest and lowest concentrations we observed among our samples were 2.14 and 0.79 mg/mL, respectively.
Figure 3: ()
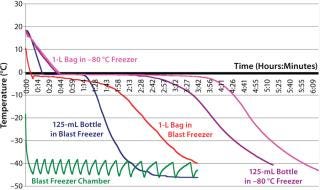
Freezing Profiles:Figure 4, shows the typical freezing profiles of a live attenuated virus vaccine DS obtained during freezing of a 6-L batch in 125 mL bottles and an 8.8-L batch in 1-L bags. The duration for phase change to occur (plateau phase, at which cooled product releases its latent heat of solidification) is commonly referred to as nominal freezing time (NFT), def ined here as the time to reach –5 °C down from 3 °C. The time taken to reach the set-point temperature from the start temperature is defined as the effective freezing time (EFT). In the bottles, the average EFT ± 1 standard deviation is 117 min ± 8 min, and the phase change (NFT) occupies ~50% of that EFT. In the bags, the average EFT±1 standard deviation is 182 min ± 27 min, and the phase-change NFT occupies ~45% of that EFT.
Figure 4: ()
Freezing times in a conventional upright freezer are much slower than those of the blast freezer. For example, the average EF Ts ± 1 standard deviation for 125-mL bottles and 1-L bags to reach –40 °C in the conventional upright freezer are 318 min ± 14 min and 339 ± 28 min, respectively. Table 1 compares specific freezing rates between the two freezers. The specif ic freezing rate (SFR) is defined here as the rate at which heat is removed from containers inside the freezer during the nominal freezing phase (from 3 °C to –5 °C) of the freezing curve. To simplify the SFR calculations, we did not take into account differences in the material and thickness of the container walls. Our results show that freezing in the blast freezer is about 2.5-fold faster than freezing in a conventional –80 °C freezer.
Table 1: Comparing specific freezing rates in a blast freezer and a –80 °C Conventional freezer
Table 1: Comparing specific freezing rates in a blast freezer and a –80 °C Conventional freezer ()
DS Potency Losses Associated with Freezing and Thawing: Following our cryoconcentration studies using the model protein solution, we used the same freezing scheme for the DS of a live-attenuated virus vaccine in 125-mL bottles and 1-L bags. To estimate potency losses, we subjected different strains of a live-virus DS to six freeze–thaw cycles with both the blast freezer and the conventional –80 °C freezer. The DS batches were 6 L distributed in 60 125-mL bottles and 8.8-L distributed in 11 1-L bags. In all cases, the thawing method was the same (as described above), and the DS contained a cryoprotectant at a minimum. We calculated potency loss per cycle in log10 infectious units/mL using regression analysis.
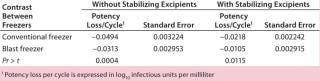
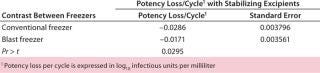
Table 2 contrasts freezing losses per cycle for both types of formulations in 125-mL bottles frozen in the blast and conventional upright freezers. The differences were statistically significant. Table 3 contrasts freezing losses per cycle for both types of formulations in 1-L bags frozen in the two freezers. The differences were statistically significant.
Table 2: Potency loss after a freeze-thaw cycle of a live-virus drug substance in 125-mL bottles
Table 2: Potency loss after a freeze-thaw cycle of a live-virus drug substance in 125-mL bottles ()
Table 3: Potency loss after a freeze-thaw cycle of a live virus drug substance in 1-L bags
Table 3: Potency loss after a freeze-thaw cycle of a live virus drug substance in 1-L bags ()
A Worthy Alternative
Vaccines have tremendous global potential for preventing and treating diseases. Efficient freezing of the vaccine DS is an essential manufacturing step in most cases to realize the full potential of vaccines to be cost effective. Because process scales and economics vary among different vaccine products, an extensive capital expenditure on freezing equipment may not always be feasible. So freezing equipment that minimizes cryoconcentration, requires relatively low capital expenditure, is easy to maintain, and can be used with disposables is highly desirable. Here we describe air-blast freezers as one such freezing option for freezing medium-scale volumes of vaccine DS in disposable containers. Among the containers evaluated in the case study presented, the maximum protein concentration variation we observed was in the two to threefold range. Further, freezing of a live-virus vaccine DS in the blast freezer resulted in little to no significant product losses. We conclude that air-blast freezers are an attractive option for freezing medium scale volumes of vaccine DS in disposable containers.
About the Author
Author Details
Corresponding author Ashish Bezawada is a process engineer, Mark Thompson is director, and Weidong Cui is principal scientist in the vaccine process biochemistry group at Medlmmune LLC; 1-650-603-2621, fax 1-650-603-3621; [email protected]; get=”new” url=”http://www.medimmune.com”>www.medimmune.com.
REFERENCES
1.) GAVI Alliance: Progress Report 2010. Global Alliance for Vaccines and Immunizations: Geneva, Switzerland, 2009.
2.) Inf luenza Fact Sheet 2003., World Health Organization, Geneva.
3.) The Vaccines Market Outlook to 2014 2009., Business Insights Ltd, London.
4.) Douglas, G, and V. Samant Plotkin, SA, WA and PA. 2008.Chapter Three: The Vaccine IndustryVaccines5th Edition, Elsevier (Saunders), Philadelphia.
5.) World Vaccines Market 2008-2013: Future Forecast 2009.Critical Trends and Developments, Renub Research Reports, Roswell.
6.) Shire, SJ. 2009. Formulation and Manufacturability of Biologics. Curr. Opin. Biotechnol 20:708-714.
7.) Wainwright, WH. 2006.The Development of Live Attenuated Rotavirus Vaccines: A Manufacturer’s Resource Guide, PATH, Seattle.
8.) Webb, S. 2002. Freezing Bulk-Scale Biopharmaceuticals Using Common Techniques and the Magnitude of Freeze Concentration. BioPharm 15:2-8.
9.) Kohle, P. 2010. Large-Scale Freezing of Biologics: Understanding Protein and Solute Cryoconcentration Changes in a CryoVessel – Part 1. BioPharm Int. 23:53-60.
10.) Wilkins, J, D Sesin, and R. Wisniewski. 2001. Large-Scale Cryopreservation of Biotherapeutic Products. Innov. Pharma Technol. 1:174-180.
11.) Singh, SK. 2007. Storage Considerations as Part of the Formulation Development Program for Biologics. Am. Pharmaceut Rev 10:26-33.
12.) Goldstein, A. 2009. Freeze Bulk Bags: A Case Study in Disposables Implementation. BioPharm Int:S4-S14.
13.) Voute, N. 2004. Disposable Technology for Controlled Freeze-Thaw. BioProcess Int. 2:S26-S31.
14.) Wisniewski, R, and V. Wu Avis, K and V. 1996.Large-Scale Freezing and Thawing of Biopharmaceutical ProductsBiotechnology and Biopharmaceutical Manufacturing, Processing, and Preservation, Interpharm/CRC Press, Boca Raton:7-59.