Challenges in Implementing Quality By Design: An Industry Perspective
June 16, 2015
In the fall of 2004, the US Food and Drug Administration (FDA) published a final report entitled Pharmaceutical CGMPs for the 21st Century: A Risk-Based Approach (1). This publication set the groundwork for a prospective risk‑based approach to pharmaceutical product development. It was published on the heels of a November 2003 agreement between the FDA and the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) to develop an internationally harmonized plan for developing a pharmaceutical quality system based on an integrated approach to risk management and science. Integral to a quality‑based system that is rooted in science and risk management is the concept of quality by design (QbD). It is a systematic approach to drug development that begins with predefined objectives and emphasizes product and process understanding and process control based on sound science and quality risk management (2).
Since 2004, a number of working groups and pilot programs have sought to “incorporate elements of risk and quality by design throughout the life cycle of the product” (1). Fast forward to 2014, implementation of QbD industrywide has been slow. But the FDA has now “strongly suggested” QbD elements, and regulatory requirements are soon to be required in generic‑drug applications. However, the pharmaceutical industry has yet to fully embrace QbD and will soon need to fundamentally change and/or evolve different modes of drug product development inline with QbD concepts. The current challenges for QbD implementation are numerous. This article serves as a QbD introduction and surveys the current state of QbD implementation. It focuses on an industry perspective with specific discussions regarding industry challenges.
Acronyms
Background
At an October 2005 workshop sponsored by the FDA and the American Association of Pharmaceutical Scientists (AAPS), FDA deputy commissioner Janet Woodcock discussed the state of drug development. She described it as “costly, wasteful, and encouraging industry to conduct more tests and file more data than needed [leading] to drug shortages, slower drug development, and intensive regulatory oversight” (3). In an effort to address those issues, the FDA established a pharmaceutical quality assessment system (PQAS) and outlined the agency’s thinking in the article Pharmaceutical CGMPs for the 21st Century: A Risk-Based Approach (1). The PQAS was designed to stimulate manufacturers to adopt modern pharmaceutical product‑development approaches leading to a desired state of drug regulation, which would result in, according to Woodcock, “a maximally efficient, agile, flexible pharmaceutical‑manufacturing sector that reliably produces high‑quality drugs without extensive regulatory oversight” (3). To that end, the concept of QbD was introduced as a means for manufacturers to achieve the desired state.
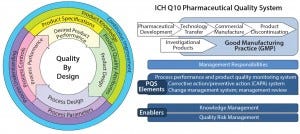
Figure 1: Quality by design embraces an integrated science and risk-based approach with continuous improvement for the entire product life cycle; PQS = pharmaceutical quality system (5, 6).
Introduction to QbD
QbD is a systematic approach to drug development that begins with predefined objectives and emphasizes product and process understanding and process control, all based on sound science and quality risk management. Moheb M. Nasr, PhD (formally of the FDA’s Center for Drug Evaluation and Research, CDER, and regarded as an agency pioneer in shaping QbD) explains that in a QbD system (4, 5)
the product is designed to meet patient needs and performance requirements
the process is designed to consistently meet product critical quality attributes
the impact of starting raw materials and process parameters on product quality is understood
critical sources of process variability are identified and controlled with appropriate control strategies
the process is continually monitored and updated to allow for consistent quality over time.
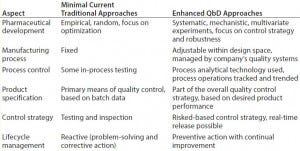
Table 1: Comparing traditional pharmaceutical development with a QbD approach (7)
Figure 1 illustrates the integrated science‑ and risk‑based approach with continuous improvement. Not so subtly, the circular paradigm emphasizes an entire product life cycle inline with ICH Q10 (6). Table 1 compares the traditional approach of pharmaceutical development to QbD and the “Frequently Used Terms” box summarizes such terms adoptable by the FDA and the ICH when describing QbD. Figure 2 presents a simplified approach to pharmaceutical development using QbD tools and risk‑based control.
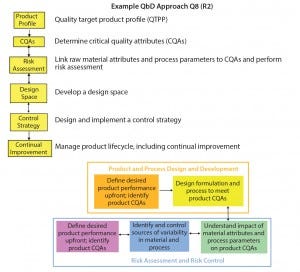
Figure 2: Simplified approach to QbD and risk control; QbD links drug quality to product life cycle by establishing a “line of sight” from desired product attributes through continual improvement (10, 11).
What QbD Is Not: QbD is not new. The concept has its roots in design of experiment (DoE), stemming from R. Fisher, who did seminal work on DoEs, at the Rothamsted Experiment Station in England in the 1920s and 1930s, and by G. Box and his colleagues in the 1950s and 1960s. QbD was popularized in the 1990s by J. Juran, founder and chairman of the Juran Institute” (10). QbD was not intended or expected to solve all pre‑QbD quality challenges. Some common misconceptions regarding QbD are
that it brings more scrutiny, more questions, and delays in approvals
that it leads to preapproval inspections and approval delays
that design space and DoE are the same
that release based on compliance with design eliminates the need for specifications
that “criticality and risk” means that if a parameter is controlled, then it stops being critical.
Although QbD holds the promise of drug development with a higher level of product quality assurance, the traditional development approach also is adequate for regulatory submission. Currently, testing and inspection ensure high product quality, but they typically require extensive regulatory oversight and substantial effort (by regulators and industry) coupled with considerable waste.
The FDA’s Implementation: The FDA has participated in various expert working groups (EWGs) and international working groups focused on implementation of QbD (ICH Q8 EWG and ICH Q9 EWG). ICH member states have finalized ICH Q8, Q9, and Q10 documents and have provided internal and external training on them. In addition, the FDA set forth various QbD chemistry, manufacturing, and controls (CMC) pilot programs (e.g., ONDQA’s CMC pilot program initiated in July 2005 and FDA’s Office of Biotechnology Products pilot program in July 2008). The agency also wrote and published mock biologic case studies (A‑Mab), and guidance documents. The FDA drafted a QbD CMC review MaPP (MAPP 5016.1, Applying ICH Q8 (R2), Q9, and Q10 principles to CMC review) and wrote and published points to consider documents.
According to the ONDQA, “implementation of QbD will enhance the assurance of pharmaceutical quality in the US market and improve the quality of CMC information submitted to the FDA in applications, supplements, and drug master files. ONDQA has been working with industry sponsors and other FDA offices to encourage the use of QbD in new drug applications (NDAs) received for new molecular entities (NMEs)” (2). However, from FDA’s perspective, challenges to QbD implementation still exist (5, 11). The “Benefits of Implementing QbD” box lists such potential benefits for both the FDA and industry. And Figure 3 illustrates a number of QbD guidances and initiatives from 2004 to 2011.
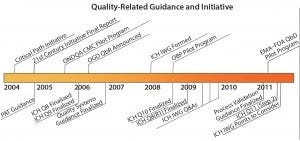
Figure 3: Quality by design guidances and initiatives timeline; regulatory assessment of applications containing QbD elements — FDA perspective (13).
An Industry Perspective
From the pharmaceutical industry’s perspective, QbD requires development of a fundamental scientific understanding of critical processes and product attributes, establishment of design controls and testing based on product quality and within the limits of scientific understanding, and use of knowledge gained over a product’s life cycle to operate in an environment of continuous improvement (14). Critical to QbD is sound science rooted in quality risk management (QRM). As stated previously, QbD concepts are not new; rather, “such innovations are the application of those principles in the development, submission, and manufacturing of drug products and drug substances” (15).
Industry’s Implementation: The biopharmaceutical industry is currently experiencing a “knowledge and experience deficit” regarding the use of QbD concepts (15). Typically, manufacturers learn through experience, and until they have successfully used QbD with positive regulatory submissions, there will continue to be trepidation about using the QbD framework in drug product development.
Frequently Used QbD Terms (References 6–9)
Quality Attribute: A physical, chemical, or microbiological property or characteristic of a material that directly or indirectly alters quality | Critical Quality Attribute (CQA): A quality attribute that must be controlled within predefined limits to ensure that a product meets its intended safety, efficacy, stability, and performance |
|---|---|
Real-Time Release (RTR): Ability to evaluate and ensure acceptable quality of an in-process and/or final product based on process data, including valid combination of assessment of material attributes by direct and/or indirect process measurements and assessment of critical process parameters and their effects on in-process material attributes | Process Parameter: An input variable or condition of a manufacturing process that can be directly controlled in the process. Typically, such parameters are physical or chemical (e.g., temperature, process time, column flow rate, column volume, reagent concentration, or buffer pH). |
Critical Process Parameter (CPP): A process parameter whose variability has an influence on a CQA and therefore should be monitored or controlled to ensure a process produces a desired quality. | Process Performance Attribute: An output variable or outcome that cannot be directly controlled but is an indicator that a process performed as expected |
Key Process Parameter (KPP): An input process parameter that should be carefully controlled within a narrow range and is essential for process performance; a key process parameter does not affect product quality attributes. If the acceptable range is exceeded, it may affect the process (e.g., yield, duration) but not product quality. | Non-Key Process Parameter: An input parameter that has been demonstrated to be easily controlled or has a wide acceptable limit. Such parameters may influence quality or process performance if acceptable limits are exceeded. |
Design Space: The multidimensional combination and interaction of input variables (e.g., material attributes) and process parameters that have been demonstrated to provide assurance of quality; working within a design space is not considered to be a change requiring regulatory approval. Movement out of a design space is considered to be a change and would normally initiate a regulatory postapproval change process. Design space is proposed by an applicant and is subject to regulatory assessment and approval (ICH Q8). | Control Strategy: A planned set of controls, derived from current product and process understanding, that ensures process performance and product quality; such controls can include parameters and attributes related to drug substance and drug product materials and components, facility and equipment operating conditions, in-process controls, finished-product specifications, and associated methods, and frequency of monitoring and control (ICH Q10). |
Quality Target Product Profile (QTPP): A prospective summary of the quality characteristics of a drug product that ideally will be achieved to ensure desired quality, taking into account safety and efficacy of a drug product |
Implementation Challenges: Waiting for the Mandate
An ever‑present theme in the pharmaceutical product development world is the FDA mandate. Industry tends to wait for an FDA mandate before fully embracing a new concept or new approach. This barrier is pervasive and supports the notion by those in industry that suggest that because regulatory agencies don’t require it, manufacturers need not adopt it. This argument misses the point: QbD is an improved approach and should be adopted before any agency mandate (3).
The current regulatory trend for required QbD elements in submissions will soon make a mandate a reality. In the broader pharmaceutical realm, as of 2013, the QbD framework for generic drug development is mandatory. For instance, according to FDA internal policy, MAPP 5016.1 (which became effective February 2011) (16), FDA CMC reviewers are instructed to review submissions for the following ICH Q8, Q9, and Q10 elements:
quality target product profile (QTPP)
critical quality attributes (CQAs) of a product
product design and product understanding
process design and understanding
product and process control strategies.
Implementation Challenges: Training and Acumen
QbD requires detailed scientific justification and rationale to link control strategy to product quality using a risk‑based approach. Implementing sound scientific principles in product and process design involves an interdisciplinary strategy using chemistry, biology, physics, engineering, math, statistics, and an ability to translate technical information into continuous improvement with innovative efforts. Most current industry decision makers have not been exposed to such a multidisciplinary approach. Additionally, most of them have long been away from academia since the conceptual application of QbD for pharmaceutical product development was incorporated in standard educational materials.
Training and consultation is intended to bridge the gap without requiring current industry managers to commit to learning and implementing QbD concepts. The transition will continue to progress slowly. A straightforward example of such a challenge is the disparate and “improper use of basic QbD terminology, such as CQAs, CPPs, and in particular, design space” within industry (17). The terms and definitions form the basis for understanding and are a prerequisite to implementation.
Implementation Challenges: Unclear Expectations
Much current FDA focus is on the effective implementation of QbD for generic drugs because the agency realizes that previous knowledge from innovator companies can be leveraged for new products, particularly for generic drugs. However, for the successful industrywide implementation of QbD, industry must “address process and product quality concerns for existing products,” NMEs, and biologics to remain “competitive and prevent problems with regulatory submissions, product recalls, and GMP compliance enforcement” (17).
Yet, unclear QbD and regulatory expectations do exist, so industry will have to work closely with the FDA to fulfill the spirit of QbD inline with the FDA’s current thinking. Case in point for unclear expectations is the application of QbD to different dosage forms such as sterile products, topical products, and oral inhalation and nasal products. Each dosage form has unique quality requirements, and the challenges are how to adapt the QbD framework and elements to those requirements. Elements such as dosage forms have received less attention and consequently less‑clear expectations (18).
Summary and DiscussIon
QbD is a systematic approach to drug development. It begins with predefined objectives and emphasizes product and process understanding and process control based on sound science and quality risk management (2). The current challenges to QbD implementation from an industry perspective are numerous because industry has yet to fully embrace its application to pharmaceutical product development.
Three of the most prevalent challenges discussed here (waiting for the FDA mandate, QbD training and acumen, and unclear QbD and regulatory expectations) highlight the need for a better understanding and an industry willing to share ideas without fear of disclosure. Modern development holds the promise of a high level of assurance of quality built on a foundation of sound risk‑based science. To continue to close the gaps between traditional and modern (enhanced) development, industry must engage the FDA. The pharmaceutical industry must deliver to expectations and strive toward innovation and creativity. The result will be further development of QbD frameworks and concepts which can play a role in setting effective agency policy and meaningful QbD‑centric regulations.
Benefits of Implementing QbD
References
1 US Food and Drug Administration. Pharmaceutical CGMPs for the 21st Century: A Risk-Based Approach. Final Report. FDA: Rockville, MD, 2004.
2 US Food and Drug Administration, Center for Drug Evaluation and Research, Office of New Drug Quality Assessment. Quality by Design Implementation: Significant Accomplishments to Date; www.fda.gov/aboutfda/ transparency/track/ucm238167.htm.
3 Wechsler J. FDA Moves to Streamline CMC Review, Promote Drug Quality. Pharma. Technol. 29 (12) 2005: 36–42.
4 Nasr MM. Pharmaceutical Quality Assessment System (PQAS) in the 21st Century. DIA Annual Meeting, Philadelphia, PA, 19 June 2006.
5 Nasr MM. Implementation of Quality by Design (QbD): Status, Challenges and Next Steps. Advisory Committee for Pharmaceutical Science (ACPS), 5 October 2006.
6 ICH Harmonised Tripartite Guideline Q10: Pharmaceutical Quality System (step 4). International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use: Geneva, Switzerland, August 2009.
7 ICH Harmonised Tripartite Guideline Q8(R2): Pharmaceutical Development (step 4). International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use: Geneva, Switzerland, August 2009.
8 ICH Harmonised Tripartite Guideline Q9: Quality Risk Management (step 4). International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use: Geneva, Switzerland, November 2005.
9 Parenteral Drug Association. Technical Report No. 42: Process Validation of Protein Manufacturing. PDA J. Pharm. Sci. Technol. 59(4 suppl. TR42) 2005: 1–28.
10 Snee RD. Building a Framework for Quality by Design. Pharma. Technol. 33(10): 2009.
11 Nasr MM. Implementation of Quality by Design (QbD): Current Perspectives on Opportunities and Challenges — Topic Introduction and ICH Update. Advisory Committee for Pharmaceutical Science and Clinical Pharmacology, 27 July 2011.
12 Winkle HN. Implementing Quality by Design. PDA/FDA Joint Regulatory Conference Evolution of the Global Regulatory Environment: A Practical Approach to Change 24 September 2007.
13 Miksinski SP. Regulatory Assessment of Applications Containing QbD Elements: FDA Perspective. Meeting of the Advisory Committee for Pharmaceutical Science and Clinical Pharmacology, 27 July 2011.
14 Kovaleski JM. Implementation of Quality by Design: Industry Perspective. DIA Annual Meeting, Philadelphia, PA, 19 June 2006.
15 Little TA. Essentials in Quality By Design. BioProcess Int. 12(3) 2014: 14–18.
16 Manual of Policies and Procedures (MAPP 5016.1): Applying ICH Q8 (R2), Q9, and Q10 Principles to CMC Review. Center for Drug Evaluation and Research Policy and Procedures, Office of Pharmaceutical Science and Office of New Drug Quality Assessment: Rockville, MD, 8 February 2011.
17 Davidson J. FDA Study on the Status of QbD Implementation in the Generic Industry. Peng D, GPhA/FDA 2013 CMC Workshop Presentation, Quality by Design for Generic Drugs, 29 January 2013; www. lachmanconsultants.com/fda‑study‑on‑the‑ status‑of‑qbd‑implementation‑in‑the‑generic‑ industry.asp.
18 Sherwood A. Quality by Design Industry Perspective. GPhA/FDA 2013 CMC Workshop, 29 January 2013.
Michael Torres is principal consultant at Thomas A. Little Consulting; 12401 North Wildflower Lane, Highland, UT 84003; 1-909-319-4521; [email protected]. The article was first written as a final project paper for MS of Regulatory Science and MS of Biotechnology (Johns Hopkins).
You May Also Like





