PHOTO BY LEAH ROSIN
On the night of Wednesday, 27 September 2017, the fourth annual Battle of the Biotech Bands raised US$110,000 for the bands’ selected charities. More than 800 guests enjoyed refreshments at the Royale Nightclub in Boston and watched Led Zymmelin (pictured right, representing Sanofi Genzyme) walk away with the top prize. They raised money for the National Organization for Rare Disorders (NORD). The crowd was a mix of Biotech Week Boston attendees; arts and entertainment critics; developers, contractors, architects, engineers, furniture dealers, and others who serve the life-science community; media; and charitable foundation representatives.
Charities supported by the other contestants included The Jolane Solomon Research Fund at Boston College (Aural Gavage, representing Momenta Pharmaceuticals) and The Michael J. Fox Foundation for Parkinson’s Research (Lifesize, representing GE Healthcare Life Sciences). Representing Blueprint Medicines, Aquinnah Pharmaceuticals, and Harmonic Lab Sol...
Revealed: Where Scientists Want to Work
When you’re in the business of life-science innovation, highly trained employees are worth every penny. That’s why biotechnology companies pay top dollar for amenities-rich locations and facilities in the top US life-science real estate markets, according to Jones Lang LaSalle’s (JLL’s)
Life Sciences Outlook
published this past summer. JLL is a professional services firm that specializes in real estate and investment management. Its report says that life-science professionals have high expectations for their workplaces.
“With Millennials often at the center of talent recruitment, life-science companies are seeking spaces that improve employee well being,” said Roger Humphrey, executive managing director and leader of JLL’s life-science group. “Some are following the lead of technology companies to create workplaces that engage, inspire, attract, and retain talent because the people are the business.”
The demand for highly skilled labor from a limited pool of candi...
Drug manufacturers are facing unprecedented serialization challenges. Serialization requires weighty consideration and focused strategy for successful commercialization, even for those companies that have yet to bring a product to market.
The World Health Organization estimates that 10% of medicines worldwide and up to 50% of drugs consumed in developing nations are counterfeit. In response to increasing drug integrity concerns, more than 40 countries have introduced laws mandating serialization and tracing of pharmaceutical products as they pass through the supply chain. Currently, over 80% of the world’s prescription medicines are expected to be protected by serialization and track-and-trace regulations by the end of 2020.
Starting in November 2017, all pharmaceutical companies selling prescription drug products in the United States are required to serialize each individual, salable drug unit to aid tracking from manufacturer to pharmacy or doctor’s office, according to the 2013 Drug Supply Chain Securi...
PALL LIFE SCIENCES (WWW.PALL.COM)
Single-use technologies offer significant advantages over traditional stainless-steel solutions for biopharmaceutical manufacturing. Reductions in setup times, cleaning and cleaning-validation costs, elimination of cross-contamination risks, and smaller footprints are just some of the benefits they provide. Although adoption of single-use systems (SUS) for commercial manufacturing is expanding, concerns persist that extractable and leachable (E&L) compounds from plastic SUS components potentially can leach into final drug products and compromise efficacy and safety. Those concerns are magnified amid the growing number of SUS suppliers and the complex supply chain for SUS and disposable components. In addition, although all equipment used for manufacturing biologic drug substances and drug products must meet current good manufacturing practice (CGMP) requirements (
1
) and be free of materials that can affect the safety or efficacy of drug products (
2
), no specific regul...
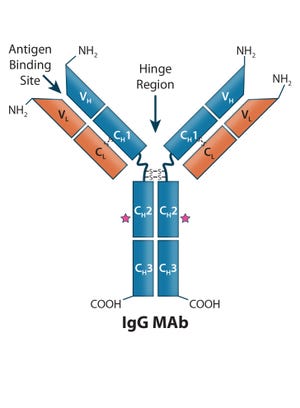
Figure 1: Human IgG1 antibody basic structure — the monoclonal IgG antibody (MAb) consists of two identical heavy chain (HC) and two identical light chain (LC) subunits. Each HC has four domains (one variable VH and three constant CH1, CH2, and CH3); each IgG LC has two domains (one variable VL, and one constant CL).
Antibody-based immunotherapy has advanced significantly since 1986, when the US Food and Drug Administration (FDA) approved the first mouse monoclonal antibody (MAb) for clinical use: Orthoclone OKT-3 (muromonab-CD3). In the intervening years, researchers have applied the tools of genetic engineering to clone immunoglobulin G (IgG) genes into a number of expression vectors. In the 1990s, the bioprocess industry was able to produce fully human antibodies in cultured cells.
As of June 2017, the FDA and the European Medicines Agency (EMA) had approved 63 and 54 MAb-based therapeutics, respectively. The global market for MAbs continues to expand dramatically, and they are now used to treat a broa...
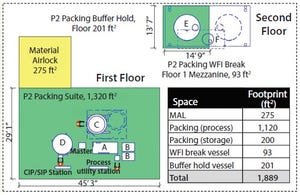
Figure 1: Modular equipment layout (A = 2-inch (235 LPM) HETP skid, B = 2 × 500-L buffer, C = 160-cm column, D = 1,600-L slurry vessel, E= 10,000-L buffer hold vessel, F = 800-L WFI break vessel)
To meet network demand for a commercial launch facility, Genentech (Roche) designed a new downstream train and built it within an existing building shell at the company’s Oceanside, CA, site. This downstream train included new technologies to allow for rapid technology transfer of different new products in the company’s drug pipeline. One technology that was pursued was the Axichrom column platform from GE Healthcare and associated column packing equipment to streamline column packing design. Here we focus on how a new approach to facility design provided flexibility, reduced capital investment, and mitigated process and start-up risks.
Flexibility
For a commercial launch facility dependent on rapid technology transfer, functionality needed to be adaptable to a variety of processing platforms as well as different...
Photo 1: Cleanroom in the Kedrion Biopharma facility contains two in-line conditioning systems with chromatography functionality.
Read this article from scientists at Cytiva (GE Healthcare at the time of publication) to learn more about buffer preparation strategies now.
Preparation and storage of buffers is a challenge for biopharmaceutical companies developing protein-based pharmaceuticals. The need for volumes of buffer to purify increasing upstream titers have become a major bottleneck in biopharmaceutical downstream processing.
Italian biopharmaceutical company Kedrion Biopharma collects and fractionates blood plasma to produce plasma-derived therapeutic products for treating and preventing serious diseases, disorders, and conditions such as hemophilia and immune-system deficiencies. To expand its offerings and include the immunoglobulin G fractionate of blood plasma (IgG, an antibody responsible for protecting against multiple bacteria and viruses), the company began construction of a new facility i...
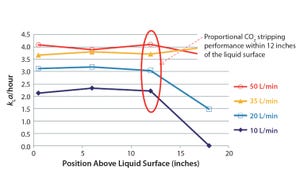
Figure 1: Crossflow sparger (with a 0.02-VVM frit) CO
2
stripping performance in a 500-L SUB
Single-use technologies (SUTs) have been adopted widely in the biopharmaceutical industry for product development as well as clinical- and commercial-scale manufacturing. Over the years, suppliers of such equipment have addressed concerns about waste management, extractables and leachables, and reliability of supply — and as a result, end users have gained confidence in SUTs. Recognizing potential benefits that can be realized for both clinical and commercial operations, biomanufacturers increasingly are implementing SU solutions at larger scales in both upstream production and downstream processing of monoclonal antibodies (MAbs), other recombinant proteins, and vaccines.
The Health Advances consultancy valued the global single-use bioreactor (SUB) market at US$220 million in 2016, projecting it to grow at a compound annual growth rate (CAGR) of 21% to reach $570 million by 2021 (
1
). An important factor drivin...
Dr. Lan Huang
Many major pharmaceutical companies — including Pfizer, Novartis, Eli Lilly, Sanofi, and Bayer — have research and development centers as well as clinical and commercial activities in China. Their sales revenues are increasing with the rapid growth of China’s healthcare market, which depending on the estimate is double or triple the country’s current gross domestic product (GDP) growth of 6.7% (
1
).
China saw 3.6 million new cancer cases in 2012, with 2.2 million cancer deaths that year. GlobalData forecasts incidents of non–small-cell lung cancer (NSCLC) in particular to rise there at 4.7% annually from 2015 to 2025, with sales of lung cancer therapies increasing tenfold. China is the second largest pharmaceutical market in the world and forecast to grow from US$108 billion in 2015 to $167 billion by 2020 (
2
).
No matter their size, all new markets pose challenges for pharmaceutical companies. Recently, a major Western drug-company executive told me that his company had spent eight years ...
In a BPI Ask the Expert webinar on 2 November 2017, Jie Chen (vice president of CMC management at WuXi Biologics) spoke about scale-out biomanufacturing strategies.
Chen’s Presentation
Scale-out strategies allow for the use of disposable bioreactors in commercial manufacturing. A scale-out strategy offers unique advantages over traditional scale-up manufacturing:
Changing market dynamics are driving a need for innovative biomanufacturing facility designs, especially for contract service providers. New facilities are focused on multiproduct and “scale-out” designs that offer flexibility, scalability, efficiency, safety, and regulatory compliance.
How can 2,000-L single-use bioreactors produce ≥100 kg? In a scale-out strategy design, multiple 2,000-L disposable bioreactors operate to provide the same volume of cell culture material as a single 10,000-L production run in roughly the same time.
WuXi Biologics has put those ideas into practice. Our 460,000-ft
2
facility is the world’s largest mammalian cell c...
byJie Chen