What we’ve come to know as the annual BioProcess International Conference has become a lot more than that. BPI magazine and conference, previously separate but collaborative business units, now live within KNect365: Informa’s relatively new business unit bringing together common interests of several new and existing businesses in the company’s “knowledge and networking” portfolio. It includes products and events from other groups that have joined Informa over the past decade or so. Some of those acquisitions may be familiar to you: IIR Holdings, the EBD group, Xconomy, and Penton Information Services.
KNect’s managing director for Life Sciences and Pharma events is Anna Chrisman, who came to Informa from EBD. It all gives us greater access to thought leaders and information than ever before, and in some areas we’ve only touched on till now. These expanded resources have transformed our business in some surprising ways. And the annual conferences (both in the United States and Europe) knit those strands in...
HTTPS://STOCK.AOBE.COM
Crowdsourcing Cancer Research and Biomanufacturing
In June, BioMed X launched two new global calls for application together with Merck KGaA for RNA splicing in cancer and for engineering of high-performance production cell lines. Talented young scientists from top universities and research institutions were invited to submit innovative project proposals and apply for a research fellowship at the BioMed X Innovation Center in Heidelberg, Germany.
BioMed X was founded in 2013 in collaboration with Merck, its first pharmaceutical partner. Since then, its innovation model has attracted sponsorship from four other companies: AbbVie, Boehringer Ingelheim, Johnson & Johnson, and Roche. Currently the organization hosts nine independent research groups from more than 20 different countries. At its innovation center, they work jointly on bioinformatics, consumer care, diagnostics, oncology, neuroscience, and respiratory projects. Four of those research groups began in collaboration with Merck...
Photo 1: Too much light behind your subject reduces your ability to see it well. A similar loss of vision occurs when organizational metrics get overcomplicated and numerous. (WWW. FREEIMAGES.COM)
This begins a five-article series of “how-to” guides for tackling the most common obstacles in assessing, measuring, analyzing, and improving the performance of global biopharmaceutical manufacturing operations. Each installment covers a component of proper collection, analysis, and use of data for the best possible performance outcomes. When taken as a whole, the series should provide imperative best practices for handling business-performance data.
First, consider what you want to know about your bioprocesses. How can you more appropriately measure those data in the right ways? “We’re drowning in data, but thirsting for information!” is a common refrain among biopharmaceutical companies when trying to understand their latest challenges. Every successful company measures a lot of things, often to a degree that ...
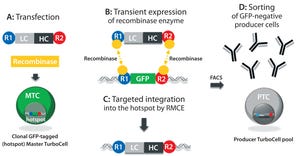
Figure 1: In the TurboCell process of rapid stable cell line development, a target vector containing the gene(s) of interest flanked by recombinase-recognition sites is transiently transfected into the GFP-expressing master TurboCell clone together with an expression plasmid for recombinase (A). After recombinase-mediated cassette exchange (B), GFP expression is lost from the GoI-expressing cells (C), which can be separated from nonrecombinated cell by FACS (D). GFP = green fluorescent protein, FACS = fluorescence-activated cell sorting, GoI = gene of interest.
In the fight against cancer, antibody–drug conjugates (ADCs) represent an increasingly important therapeutic approach. These biopharmaceuticals are designed to maximize the therapeutic index of cytotoxic small-molecule drugs through their selective delivery to tumor cells while leaving normal, healthy cells untouched. Structurally, an ADC is a monoclonal antibody (MAb) conjugated by a chemical linker to a potent cytotoxic drug. Conceptually, the MA...
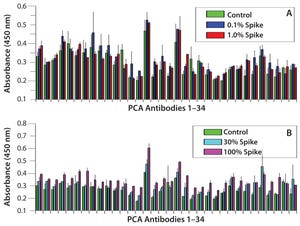
Figure 1: Refolding analysis of serum-derived human IgG after 8 M urea treatment; (A) spike study with IgG derived from human serum — urea-treated IgG spiked into native IgG solution at 0.1% and 1% and compared with native IgG only sample; (B) spike study with IgG derived from human serum — urea-treated IgG spiked into native IgG solution at 10% and 100% and compared with native IgG only sample.
Note: Not until 100% unfolded antibody is added do we observe HOS differences, which suggests that IgGs derived from human serum have a much faster refolding capability than CHO-derived proteins. Error bars show assay variation.
For protein therapeutics and other biologics, the importance of the molecule’s structure to its efficacy and safety is well established (
1
–
5
). In particular, their tertiary and quaternary structures play very important roles in product quality and have been monitored extensively in comparability studies (
6
–
12
). However, because of both the large molecular size and rotational prope...
The boundaries of technology can be pushed significantly when insights from different fields reinforce each other. Based on in silico simulations demonstrating the importance of order on the efficiency of chromatographic separations, PharmaFluidics has combined expertise from the analytical chromatography and semiconductor chip manufacturing industries to create a new type of nanoscale liquid chromatography (LC) column.
Conventional LC columns contain randomly packed beads as a stationary phase. By contrast, PharmaFluidics uses a lithographic etching process to create a perfectly ordered separation bed on a silicon chip. This new type of microchip-based chromatography column is a micro Pillar Array Column (µPAC) device.
A low “on-column” dispersion comes with the perfect order, which virtually eliminates axial peak dispersion. That provides for higher column-plate numbers with sharper peaks and higher concentration of compounds than would be otherwise possible. The freestanding nature of the pillars also ...
Photo 1: An ambr 250 modular eight-bioreactor system
Mammalian and microbial protein production platforms have been used for over 30 years to produce a number of successful biologic drugs, including monoclonal antibodies (MAbs), recombinant proteins, and therapeutic enzymes (
1
). Most biologics are produced by mammalian cell lines, with Chinese hamster ovary (CHO) cells being the most widely used. However, microbial cells also are used to express recombinant therapeutic proteins, and almost 30% of currently approved biologics are produced by
Escherichia coli
bacteria (
2
). With worldwide biologics sales >56 billion Euros per year (
3
), both industrial and academic bioprocess scientists have to develop methods for effective process development of such proteins to make them competitively priced and maintain profitability in this lucrative market.
One strategy is to work with user-friendly single-use, miniaturized bioreactors to shorten process development times. That increases efficiency and reduces th...
The out-of-pocket price of many life-saving cancer medications continues to rise while insurance companies continue to raise deductibles and copays. Patients are paying more for their prescriptions than ever before, and they need solutions that offer cost-effective treatment.
The Oncological Problem
Cancer is the second leading cause of death in the United States. More often than ever before, patients obtaining potentially life-saving cancer drugs face a severe financial burden (
1
). Newer cancer medications can cost patients over US$100,000 each year, and oncology patients commonly spend an average of $8,700 each month on those they need to fight their disease (
2
,
3
). Rising insurance deductibles and copayments force many cancer patients to pay more out-of-pocket costs for the expensive medications that could save their lives (
2
,
5
). In response, payers are calling for drug-cost solutions that give patients a more affordable way to receive the treatments they need.
Only 50 years ago, it was comm...
In an “Ask the Expert” webinar on 13 September 2017, Gerald Platteau of JSR Life Sciences described the use of Amsphere A3 resin to purify antibody fragments. He explained the molecular binding mechanism for VHH singledomain antibodies and compared dynamic binding capacity (DBC) data with those of other affinity resins.
Platteau’s Presentation
For full-size monoclonal antibodies (MAbs), the standard capture step is based on protein A. Its binding to the Fc region has been well described as taking place at the CH
2
–CH
3
interface primarily through hydrophobic interactions. Because antibodies in the same subclass have >95% homologous Fc regions, protein A affinity is a suitable capture platform for a wide antibody range.
Antibody fragments are becoming an important therapeutic class comprising a variety of molecules such as VHH single domain antibodies, Fabs (antigen-binding fragments), single VH domains, and scFvs (single chain variable fragments). Because they lack Fc regions, these antibody fragments c...
In BPI’s “Ask the Expert” webcast on 4 October 2017, Mike Johnson (market development manager at Entegris Life Sciences) discussed single-use bags and gas permeation.
Johnson’s Presentation
Permeation rates are a function of fluid properties, the type of material being penetrated, and application temperature. Standardized test methods used to determine permeability characteristics include ASTM D1434 and ASTM D3985. A fixture in Entegris’s test apparatus holds a sample plaque that is flanked upstream by high-pressure gas and downstream by a sensor that measures breakthrough time and the rate of permeation through the material.
In most single-use bags, an ethylene vinyl alcohol (EVOH) layer provides a gas-permeation barrier. The outer layer is ethylene vinyl acetate (EVA), polyethylene, or another polymer. That combination of layers raises the risk of detrimental extractables because of adhesives that bond them together. Such constructions are susceptible to delamination and film failure because of differen...