August 2017 Featured Report
This special insert summarizes our well-attended BioProcess Theater at the BIO International Convention in San Diego this past June. Focusing on emerging therapies and technologies enabled us to touch upon interconnected topics of interest to both BPI readers and the BIO exhibit-hall attendees. Speakers highlighted approaches ranging from analytical development to evolving business and commercial models. On behalf of our publisher, Brian Caine (who creates these programs every year) and our marketing and editorial teams, I thank the speakers and moderators who helped us create another successful program. They are passionate about supporting innovation in the industry and volunteer their time for us during an already full week of meetings and networking.
These are editorial summaries of the presentations. Choosing what to highlight in the allocated space is not easy! So I am very happy that the full presentations — videos so you can follow along with the slides — are online at www.bioprocessintl.com/BIO201...
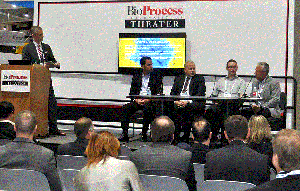
Left to right: Patricia Seymour, Richard Snyder, Paulo Carvalho, Holger Wesche, and Marc Better (PHOTO BY LEAH J. ROSIN)
At noon on Tuesday, 20 June 2017,
BioProcess International
presented a panel discussion as part of the “Emerging Therapies” session of its BPI Theater at the Biotechnology Innovation Organization’s annual convention in San Diego.
Moderated by Patricia Seymour (senior consultant at BioProcess Technology Consultants), this panel comprised Holger Wesche (vice president of research at Harpoon Therapeutics), Richard Snyder (chief scientific officer of Brammer Bio), Marc Better (vice president of product sciences at Kite Pharma), and Paulo Carvalho (associate director of manufacturing at Alnylam Pharmaceuticals).
Emerging therapies — using cell and gene therapy, ribonucleic acid interference (RNAi), T-cell engagers, and others — present new and unique manufacturing challenges for biopharmaceutical companies. These include process development and manufacturability, access to manufacturing fa...
Presented by
Sean Kevlahan
(chief executive officer, Quad Technologies) 11:00–11:20 am
Kevlahan described Quad Technologies as an early stage company founded in 2013 and headquartered in Woburn, MA. Its approach to cell and gene therapies is offering bioprocessing tools with a biomaterials focus. The company’s QuikGel platform is a hydrogel-based technology for making different sizes of hydrogel microbeads that will dissolve under gentle conditions. The beads can be magnetic or nonmagnetic for both research and bioprocessing applications. This technology provides Quad customers with a gentle and scalable solution for, e.g., T-cell separation, activation, and expansion.
The challenge in dealing with cell therapy products comes from a range of manufacturing issues. Kevlahan’s company address those with biomaterials. The QuikGel platform allows for capture and release of cells from substrates without including deleterious agents such as enzymes. The hydrogel polymer disassociates with ease. Within five sec...
Presented by
Yan Zhi
(program design technical lead, Fujifilm Diosynth Biotechnologies) 11:20–11:40 am
Fujifilm Diosynth Biotechnologies Texas (FDBT) has two dedicated viral products facilities: The National Center for Therapeutic Manufacturing (NCTM) building was designed for process development and early phase clinical good manufacturing practice (GMP) production of advanced therapeutics including viral, microbial, and plasmid products. The newly built Flexible Biomanufacturing Facility (FBF) is designed for clinical and commercial GMP production of viral products.
The NCTM boasts 14 first- and second-generation mobile cleanrooms. For upstream production cell culture, it has 10-L, 50-L, and 200-L single-use bioreactors and plans to add a 500-L unit. For fermentation, the company has 10-L and 50-L fermentors and 200-L dual-purpose single-use bioreactors. On the downstream side, the facility uses depth filtration for harvest and prepacked columns and GE Healthcare Ready2Process chromatography columns fo...
Presented by
Stewart McNaull
(senior vice president of business development, KBI Biopharma) 11:40 am–12:00 pm
Founded in 2004, KBI Biopharma operates a core facility in Durham, NC. In 2013, the company needed more space and set up laboratories in Research Triangle Park, NC. Using mammalian cell lines, it has developed both monoclonal antibody (MAb) and non-MAb platforms. It acquired a former Merck site in Boulder, CO, in 2014 and retained staff experts in fermentation expression, refolding, and mass spectrometry. With manufacturing of clinical and commercial products in 1,500-L stainless steel bioreactors, the site is building a 300-L single-use fermentation suite that will be ready this autumn. Its world-class particle characterization group and modeling–simulation group also works with the University of Boulder. In February 2017, KBI acquired Opexa Therapeutics in Woodlands, TX, where staff perform phase 1 and 2 manufacturing of cell therapy products (including cell-based assays).
Analytical, Formulat...
Presented by
Cyril Peter
(senior proposal manager, Lonza Pharma and Biotech) 1:00–1:20 pm
A publicly traded company, Lonza focuses on drug sponsors that want to go to market with their own products. “To get there, you need to make a drug out of your molecule, manufacture it, go through clinical trials, and generate data for submission to regulatory authorities for market authorization.” That requires information about the molecule (critical quality attributes, CQAs) and on how you make it (critical process parameters, CPPs). If you work with a contract development and manufacturing organization (CDMO), those items also help set roles and responsibilities each company will have. A drug sponsor (market authorization holder) owns the knowledge on safety, identity, efficacy, and quality — including identity, purity, and biological activity. A CDMO owns the manufacturing process and provides the evidence of its suitability along with that of analytical methods and equipment used. Regulatory documents make cl...
Presented by
Adam Elhofy
(chief science officer, Bio-Ess Laboratories) 2:00–2:20 pm
“What we call biomanufacturing,” said Elhofy, “is all about cells. When cells get cranked up, they start to have deficiencies — and those deficiencies lead to different protein quality, to aggregates, and to a loss of consistency in protein quality. So how do you improve productivity and protein quality?” Bio-Ess Laboratories (formerly Essential Pharmaceuticals) has a technology to address the role that lipids play in cell productivity.
The company has existed for over a decade, making media products and medical devices. Cell-Ess medium is made to good manufacturing practice (GMP) standards and is free of animal components. It is chemically defined and can be used to increase titers in serum-free systems or to replace serum in other systems.
Proteins are formed on the lipid membranes of each cell’s endoplasmic reticulum (ER), then transported to Golgi apparati for posttranslational modification on another lipid membrane....
Thomas Page
(vice president, engineering and asset development, Fujifilm Diosynth Biotechnologies USA) 1:00–1:20 pm
Page introduced Fujifilm Diosynth’s Saturn monoclonal antibody (MAb) platform, which is based on the Apollo cell line. A senior team working from three facilities designed this new platform by challenging everything from previous designs, keeping the best aspects, and throwing out what did not work well — while innovating where necessary. All functional units were represented and empowered to make decisions. The team used risk-based tools to arrive at its design.
The Project Saturn mission statement is “to enhance FDB’s global capability by offering competitive MAb development and manufacturing capacity meeting regulatory standards and client needs for rapid and reliable clinical and commercial supply.” Through this project, the company offers its clients capability, capacity, and flexibility.
Page showed a video detailing the project. The video described FDB as a current good manufacturing...
On Tuesday, 20 June 2017,
BioProcess International
presented a panel discussion from 1:20 to 2:00 pm as part of the “Emerging Therapies” session of its BPI Theater at the BIO annual convention in San Diego.
Left to right: Gil Roth, Michael Riley, John Foy, Mark Wright, and Victor Vinci (PHOTO BY LEAH J. ROSIN)
Moderated by Gil Roth (president of the Pharma and Biopharma Outsourcing Association), the panel comprised John Foy (vice president of business management for biologics at Patheon), Michael A. Riley (vice president and general manager of Catalent Biologics), Victor Vinci (vice president and chief scientific officer of Cook Pharmica), and Mark Wright (site lead for antibody–drug conjugates at Piramal Pharma Solutions).
Development and manufacture of emerging therapies follows a path similar to that of protein-based therapies. But many unique equipment, technology, logistic, and scientific challenges must be addressed early in these processes to increase their chances of commercial success. Contract...
On Tuesday, 20 June 2017,
BioProcess International
presented a panel discussion from 2:20 to 3:30 pm as part of the “Emerging Therapies” session of its BPI Theater at the BIO annual convention in San Diego.
Moderated by David Brindley (University of Oxford and Harvard University), the panel comprised Morrie Ruffin (managing partner of the Alliance for Regenerative Medicine), David Backer (head of commercial development for gene editing and novel modalities at MilliporeSigma), Robert Preti (president and chief executive officer of PCT, a Hitachi Company), and Lynne Frick (vice president of business development of IsoPlexis).
After many trials, errors, and milestones, regenerative medicine has become a mainstream part of the biologics industry supported by at least 670 companies and clinics of all sizes. This panel introduced and discussed key lessons to be gleaned from the evolution of bioprocessing to support the optimal future development of regenerative medicines; reviewed rapid changes in bioprocess ...
On Wednesday, 21 June 2017,
BioProcess International
presented a panel discussion from 11:00 am to 12:00 pm as part of the “Emerging Techniques and Technologies” session of its BPI Theater at the BIO annual convention in San Diego.
Left to Right: Stewart McNaull, Todd Menkhaus, Tom Ransohoff, and Bernard Huyghe (PHOTO BY LEAH J. ROSIN)
Moderated by Tom Ransohoff (vice president of BioProcess Technology Consultants), the panel comprised Stewart McNaull (senior vice president of business development at KBI Biopharma), Bernard Huyghe (senior director at Pfizer), John Bonham-Carter (director at Repligen, not pictured), and Todd J. Menkhaus (cofounder of Nanopareil).
With omnipresent demands to improve development speed, reduce costs, and improve product quality, the biopharmaceutical industry needs to evaluate and adopt new technologies for manufacturing and testing its products. In addition to the development costs and resources of implementing new technologies, significant regulatory, technical, and time ...
At noon on Wednesday, 21 June 2017,
BioProcess International
presented a panel discussion as part of the “Emerging Techniques and Technologies” session of its BPI Theater at the BIO annual convention in San Diego.
Left to Right: Susan Dexter, Magnus Schroeder, Deborah Harris, and Stephen Lam (PHOTO BY LEAH J. ROSIN)
Moderated by Susan Dexter (managing director of Latham Biopharm Group), this panel comprised Deborah Harris (vice president of operations at Abzena), Magnus Schroeder (director of downstream process development at CMC Biologics), and Stephen Lam (vice president of operations at Patheon).
There is no debate: Single-use technologies (SUTs) have become essential mainstream tools to lower costs and shorten time to market, extend the viability of smaller-indication products, and provide flexibility in where and how products can be manufactured. The question now is what technologies are best suited to a given bioprocess. This panel addressed factors behind related decisions and asked whether, in h...
On Wednesday, 21 June 2017,
BioProcess International
presented a panel discussion from 1:20 to 2:30 pm as part of the “Emerging Techniques and Technologies” session of its BPI Theater at the BIO annual convention in San Diego.
Left to right: Joshua Speidel, William Taylor, Diane Retallack, and Rachel Felberbaum (PHOTO BY LEAH J. ROSIN)
Moderated by Joshua Speidel (managing director of the commercial practice team at Latham Biopharm Group), this panel comprised Diane Retallack (senior director of upstream processing and intellectual property at Pfenex), Rachel Felberbaum (senior director of business development at Protein Sciences Corporation), and William Taylor (vice president of business development at Protalix BioTherapeutics).
Platform approaches to vaccine drug-substance manufacturing play a key role in accelerating the product development cycle time. The benefits are clear: Patients have earlier access to treatment, and product sponsor investments are derisked from regulatory and manufacturing per...