January 2017 Featured Report
Single-step harvesting of a high-density cell culture from a Biostat STR bioreactor with Sartoclear Dynamics body-feed filtration from Sartorius Stedim Biotech (WWW.SARTORIUS.COM)
Downstream processing has been considered a “bottleneck” in the manufacture of protein biotherapeutics ever since cell culture engineers began dramatically improving production efficiencies around the turn of the century. And as single-use technologies have grown in importance and acceptance, offering more solutions every year, their biggest challenges too have been in the separation, purification, and processing that follows product expression in cell culture. Many of the technologies familiar to process engineers — e.g., centrifugation and chromatography — present technical and economic problems.
In a recent white paper (
1
), BioPlan Associates reported that respondents to its 13th annual survey of biomanufacturers reported downstream processing as requiring the most technological improvement of any part of bioprocessing. But...
Depth filtration at Rentschler (WWW.RENTSCHLER.DE)
For current process development phases, many biomanufacturers’ attention is directed increasingly to the first unit operation in downstream processing, which is the removal of cells and cell debris from culture broth and clarification of supernatant containing a biopharmaceutical product. Given the high cell densities achievable with both mammalian and microbial cell culture processes, primary recovery can be a significant challenge.
The current trend in cell culture is to increase product titers with enriched culture media, improved cell productivity, and increased cell mass. High titers also can be achieved through increased culture duration, which can lead to a significant drop in cell viability. These factors cause the increase of process impurities such as host-cell proteins (HCPs), nucleic acids, lipids, and colloids, as well as generation of a broad particle-size distribution in cell culture fluids. Downstream chromatography requires a fast and rel...
PALL LIFE SCIENCES (WWW.PALL.COM)
Since 2001, global contract development and manufacturing organization (CDMO) CMC Biologics has completed more than 120 projects with at least 100 pharmaceutical partners. During that time, the company has taken a holistic approach to helping clients balance manufacturing risks and rewards. The team focuses on evaluating key technologies to deploy a constantly evolving set of capabilities in support of biopharmaceutical clients throughout their product lifecycles. Part of that commitment is continually evaluating what would best benefit customers and where key challenges lie.
For monoclonal antibodies (MAbs) specifically, the downstream processing group recognized a need to find alternative processing methods to reduce the cost of protein A capture chromatography. Because of its cost, this step often can be a financial barrier for some pharmaceutical innovators looking to get new drugs to the clinic. When evaluating options, it became clear that continuous, multicolumn si...
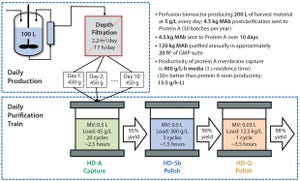
Figure 1: Right-sizing and rapid multicycling enables small-footprint downstream processing to match high-productivity upstream production (adapted from reference
1
).
Filtration membranes are used extensively throughout the biopharmaceutical industry for a range of applications, from coarse filtration to nanofiltration. Advantages of filter technologies include easy scaling, disposability, and (for many membrane filters) rapid and robust performance in a single-pass. The same advantages have been realized with membrane adsorbers.
Chromatography resins are inherently disadvantaged by diffusion limits of the pores in chromatography media. Therefore, resin columns must be significantly oversized to match the performance of high productivity bioreactors. By comparison, membrane adsorbers take advantage of filter performance to deliver highly productive downstream operations. Surface-functionalized membranes from Sartorius Stedim Biotech (Sartobind Q, STIC) and Pall Life Sciences (Mustang Q)typically use ani...
I talked with NNE Pharmaplan’s Kim Vincent Andersen (single-use technology and biotechnology specialist) and Niels Guldager (global technology partner in biotech) to discuss their experiences with client facilities that incorporate significant elements of single-use technology. In particular, they highlighted a recent project for Novo Nordisk involving a large-scale greenfield filling and inspection facility in Hillerød, Denmark. Find more detailed information about the project online at https://goo.gl/yp4LQh. And you can watch a video about it here: https://youtu.be/czwwgdt3CxI.
A Case Study
You are leading the design of a fill–finish facility that applies single-use technology in critical process steps. Can you describe the project and its application of disposables?
Andersen:
The facility is a US$300 million, 10,000-m
2
insulin filling facility currently under construction by Novo Nordisk in Denmark. It will produce commercial supply of insulin products for the global market.
Guldager:
We are workin...