March 2012 Supplement March 2012
Bioassay development is foundational to the well-characterized biotechnology product paradigm. Bioassays are the best tools for drug developers to use in determining the biological activity (potency) of their products, which has been a biopharmaceutical critical quality attribute (CQA) since long before that concept had a name. Thus, these assays are vital to quality assurance and quality control (QA/QC), preclinical studies, and clinical testing — and by extension to process development and monitoring.
Because of their complex nature, bioassays are among of the most challenging experiments to perform reliably with dependably accurate results. Consistency requires a controlled environment and qualified reagents, skilled analysts, and assay protocols that are intelligently developed, characterized, and validated. Depending on the use of a method, it could be required to comply with good laboratory practice, good clinical practice, or good manufacturing practice (GxP) regulations as well as electronic data ...
Cell-based potency testing provides quantitative data concerning a drug’s biological activity. Thus, it plays an essential role in biopharmaceutical quality control (QC), good manufacturing practice (GMP) product release, comparability determination, and stability testing for both drug products and drug substances. Potency is a critical quality attribute (CQA) often scrutinized by regulators and reviewers. Test methods are specific to a drug’s mechanism of action (MoA) and should be validated to internationally harmonized regulatory standards (
1
). The options preclude applying a simple one-size-fits-all template for automation of potency assays — typically enzyme-linked immunosorbent assays (ELISAs) — but the concept is well worth consideration.
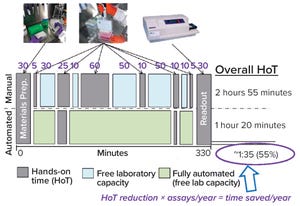
Figure 1: Efficiency gains provide time savings, but not in a simple way for potency assays; e.g., incubation times don’t change; HoT = hands-on time.
At the CASSS Well-Characterized Biotechnology Products (WCBP) conference in May 2019, Hermann Beck (F. Hoffmann...
Host-cell proteins (HCPs) are major impurities of concern in biomanufacturing. When present in drug formulations, they can reduce efficacy (by compromising product stability), introduce toxicity, and increase a recipient’s risk for long-term immunogenicity. Understanding HCP profiles and integrating effective removal strategies are important parts of developing new biological drugs — to fulfill regulatory guidelines and to ensure patient safety through product quality.
HCP populations can be both complex and structurally diverse, and some changes in upstream culture conditions can affect their concentrations and thus influence how control strategies work. Accurate and reliable HCP quantitation is essential to monitoring the effects of process adjustments and for optimizing purification steps to ensure adequate removal of impurities.
Removal of HCPs from drug substances is critical in manufacturing high-quality drug products. It is predicated on thorough analysis of HCP contaminants, which vary considerabl...
Many physical and chemical factors can affect the quality, safety, and efficacy of biopharmaceutical products, particularly after long-term storage in a container–closure system that can be subject to variations in temperature and light, as well as agitation with shipping and handling. Proteins are inherently complex physiochemically, from their primary amino acid sequences to their higher-order structures, and they require specific conditions to maintain their integrity and functionality. Advanced biological therapies can be even more complicated and particular about their environments.
Stability testing is used to detect changes in identity, purity, and potency of a formulated drug product over time. Stability assays are used throughout each product’s life cycle, beginning with development and performance of comprehensive and specific stability protocols during preclinical development and early clinical phases. Under the quality by design (QbD) paradigm, stability is part of a biotherapeutic’s quality t...
Subscribe and receive the latest insights on the healthy food and beverage industry.
Join 47,000+ member. Yes, it's completely free.