www.photos.com
Here we present a consensus position of the membership of the Bio-Process Systems Alliance (BPSA), the trade organization for the single-use industry based in Washington, DC. BPSA’s membership includes 48 corporate and institutional entities, among them component suppliers, systems integrators, end users, and independent testing laboratories. Consensus within this membership is reached through an official ballot of representative voting members, as provided for in the organization’s by-laws. The position outlined below was approved by such an internal consensus-balloting process.
Building Consensus
Single-use systems (SUS) have found broad acceptance in pharmaceutical manufacturing based on their operational and economic benefits. To expedite selection and implementation of such systems, industry-wide discussions have been ongoing for several years with the goal of aligning basic qualification requirements. In particular, the development of consensus thinking on extractables testing for co...
An extractables profile offers comprehensive knowledge of the materials involved in the
packaging components that are in direct contact with a drug product.
Biopharmaceutical manufacturers spend years developing and testing new drug and biologic products to ensure their efficacy, safety, and usability for patients. Such knowledge is extremely valuable, but those same principles often get overlooked in selection of packaging and delivery systems. Decisions on packaging and delivery often are made almost as an afterthought. However, issues related to components such as extractables and leachables can affect patient safety and product quality. In addition, a lack of extractables and leachables data in filings with the US Food and Drug Administration can delay therapeutic approval, wasting a company’s valuable time and money.
An extractables profile demonstrates comprehensive knowledge of the materials involved in packaging components that come into direct contact with a drug product. Extractables testing tak...
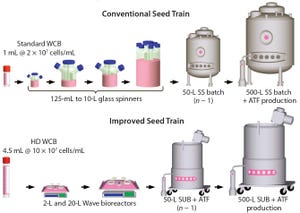
A typical cell culture process begins with thawing of a cryopreserved cell-bank vial, followed by successive expansions into larger culture vessels such as shake flasks, spinners, Wave bags, and stirred bioreactors (
1
). When culture volume and cell density meet predetermined criteria, the culture is transferred to a production bioreactor in which cells continue to grow and express product. This approach presents several challenges.
Figure 1:
Comparing a conventional with an improved seed-train process for inoculation of a
500-L perfusion bioreactor; the new process uses high-density (HD) cell banking, single-use
technology, and high-density perfusion at the n – 1 stage to allow for high-density inoculation in
the production bioreactor. HD cell banking and disposables improve operational success by
reducing seed-train complexity and contamination potential. HD perfusion at the n – 1 stage allows
for HD inoculation of the production bioreactor, reducing the time to steady-state cell density by
4–5 days a...

 +8
+8EB66 cells
VALNEVA SE (WWW.VALNEVA.COM)
Cell substrates managed in controlled culture environments have become, over the past few decades, the subject of intensive technological developments for the biomanufacturing of viral vaccines. The driving force of such work is an expanding demand for safety, high production capacities, cost savings, and flexibility. Egg, tissue, and primary-cell–based manufacturing methods of limited capacity are now considered to be outdated technologies.
In the influenza vaccine field, for example, time delays in vaccine delivery (especially during pandemic responses) have increased concerns over whether the classic egg-based production system is capable of meeting increasing demands. Hence, manufacturing with cell culture platforms offers high flexibility and optimal scaling capacities, important features for guaranteeing vaccine supply.
Demonstrating the expanding interest for such technologies, several cell-based influenza vaccines have been recently approved for market (
1

 +4
+4Much has been written in recent years about the union of single-use systems (SUS) and process automation. These two technology initiatives have been prevalent within biopharmaceutical manufacturing over the past decade and are two of the most predominant advancements in biomanufacturing. A basic survey of industry media and conference topics corroborates that premise (
1
). However, efforts to combine them remain in the early stages of technological fulfillment, with much work to be done in realizing their synergistic benefits. Here I review the current state of this amalgamation of SUS and process automation, discuss possibilities moving forward, and look at the merits thereof.
Divergent Outputs
Each year, many conferences, publications, and events are devoted to SUS and process automation in biomanufacturing. Those focusing on SUS often are most easily recognizable because they feature either “Single- Use” or “Disposable” in their titles, but those focusing on process automation can be more inconspicuo...
Single-use technologies have been implemented in biomanufacturing facilities all over the world. Inherently more flexible than stainless steel equipment, single-use technology allows for more rapid technology transfer by minimizing the time it takes to design, purchase, and qualify new capital assets. Rapid turnover between batches is facilitated with no need for protracted clean-in-place (CIP) and steam-in-place (SIP) regimes; the risk of product cross contaminations is reduced because single-use fluid-contact surfaces are never previously exposed to a biopharmaceutical product stream. For those reasons and more, single-use technologies have been widely adopted within the industry, especially by leading contract manufacturing organizations (CMOs).
Different levels of automation are available for those implementing single-use solutions, from completely manual steps through semiautomated to completely automated unit operations. The industry seems to a have a preference for semiautomation, in which a degree...
Measuring pressure in single-use systems (SUS) has become an integral part of both upstream and downstream bioprocess operations. Articles have been published on filtration applications (
1
), and integration into other SUS has been widely adopted. Additionally, information is available on low-pressure applications such as how to prevent overpressurization in single-use bioreactors (
2
).
Photo 1:
Single-channel PressureMAT unit for
low-pressure monitoring
However, as users and applications both become more sophisticated, improved performance is sought for low-pressure applications (<1 psi) such as in single-use bioreactors. The reasons are two-fold: First, more information can be gleaned through improved accuracy in measuring a SU process condition. Second, with improved performance, useful applications such as leak testing and pressure hold can be better evaluated for implementation in SUS by using a sensor installed on a given system for in-process monitoring.
Single-use pressure sensors such as Pendo...

 +2
+2Although significant scientific progress has been made in the biotechnology industry, it has lagged behind other sectors — such as aviation and automotive — in developing, scaling up, and industrializing products coming out of R&D. The goal is to implement robust and reliable manufacturing processes for good manufacturing practice (GMP) market supply cost-effectively. But manufacturing methods for biologics have remained unchanged for decades, with large-scale, capital-intensive stainless steel facilities taking three to five years to build and remaining both energy intensive and expensive to operate. When you take into the account a strong attrition rate through the different clinical phases for biologics and the fact that many drugs do not make it to market, that business model always represented high risk because such facilities were fixed, with little or no flexibility for redesign if a drug didn’t make it to market
Over time, the industry has come to realize that such a business model is no longer su...