Not too long ago, many bioprocessing professionals perceived the annual BIO International Convention as an event about biotechnology business, financial matters, investing, and partnering — and until not too long ago, for the most part, that was correct.
In 2007, the Biotechnology Industry Organization and
BioProcess International
formed a partnership to create a dedicated destination where bioprocessing professionals could learn about the latest technologies and trends in biopharmaceutical development and manufacturing. Thus was born the BioProcess Theater and Zone.
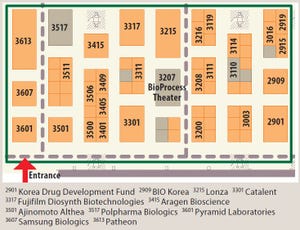
Every year since then, leading biopharmaceutical suppliers have exhibited in the BioProcess Zone to showcase their experience, innovations, and services to a BIO audience. The BPI Theater is a dedicated amphitheater located on the exhibition floor at the heart of the BioProcess Zone (Figure 1). It is designed to supplement BIO’s main educational program and provide convention attendees with complementary scientific programming every day — w...
BIO is the world’s largest trade association representing biotechnology companies, academic institutions, state biotechnology centers and related organizations across the United States and in more than 30 other nations. BIO members are involved in the research and development of innovative healthcare, agricultural, industrial, and environmental biotechnology products. BIO plays a leading role in shaping public policy related to the biotechnology industry — at the state, national, and international levels.
Jim Greenwood has been BIO’s president and CEO for 10 years, during which time he has increased the trade association’s staff and budget by nearly 50%. Before his BIO appointment in January 2005, Greenwood represented Pennsylvania’s eighth district in the US House of Representatives (1993–2005). From 2001 to 2004, he served as chair of the Energy and Commerce Committee Subcommittee on Oversight and Investigation, leading investigations into corporate governance, terrorist threats to the nation’s infrastr...
BioProcess International
launched about the same time as a major FDA regulatory announcement that has radically altered biopharmaceutical development: The quality by design (QbD) initiative is an important part of the agency’s 21st-century good manufacturing practice (GMP) approach, which is changing how regulators review product applications and thus how companies must approach them (1). It has placed increasing pressure on analytical laboratories, whose work is more important to the success of biotherapeutic products than ever before.
Backed by harmonized tripartate guidelines from the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH), the guiding principles of QbD include risk management, science-based policies and standards, and integrated quality systems. International cooperation brings Europe, North America, and parts of Asia together in agreement on the value of this approach to public health protection.
QbD particularly em...
Process development forms the core of
BioProcess International’s
coverage and interests. From cell-line engineering through seed-train and production cultures, the results of which are harvested and purified to yield a bulk drug substance, every biologic therapy and vaccine begins its life at small scale in a research and development laboratory.Cell lines may be mammalian, insect, microbial, or even plant-sourced (1). They all require different types of culture media and supplements. And purification options are many and diverse. Process intermediates are collected and stored along the way, as are final bulk substances ready for formulation into drug products.
Whatever variations a specific product requires of its biomanufacturing process, all biologics must go through a series of well-documented stages with increasing regulatory stringency. Under the 21st-century regulatory paradigm, bioprocesses must be engineered to produce high-quality products by design. New technologies such as single-use systems ...
On Tuesday 16 June 2015, at noon on the BioProcess Theater stage at BIO 2015, Tom Ransohoff (vice president and principal consultant at BioProcess Technology Consultants, Inc.) will moderate a roundtable discussion with the following panelists:
The world of biomanufacturing is more dynamic than ever: Witness the rise of biosimilars, the return of vaccines, and the emergence of new types of products. Meanwhile, process improvements march on, producing higher upstream titers and greater downstream efficiencies. The overall goal remains the same: faster product development at lower cost with better quality. This panel will review emerging strategies for achieving that goal in a competitive global environment. Participants will focus on process intensification (e.g., continuous processing, real-time release, and integrating single-use components).
The Modera
t
or Speak
s
BPI’s online editor, Leah Rosin, spoke with Ransohoff in March 2015.
Can you tell us about the panelists you might be inviting to the BIO Th...
Fragile proteins and other biomolecules need protection as stable drug products. The larger a molecule is, the more difficult it will be to make, ship/store, and administer to patients. Biotech drug formulators have many concerns to juggle in their work, beginning with the physicochemical characteristics of an active molecule and including the reliability, cost, and availability of analytical methods; the array of excipients and adjuvants on the market; evolving delivery methods and devices; patient preferences and behavior, as well as the biology of diseases being treated; and even the concerns of legal, sales, and marketing groups.
Formulation is more than science, as a result, but science is its foundation. For years, it has been considered by many as an arena where luck and intuition play a role as well. But the work has become more methodical and quantifiable with new analytical technologies and the advent of quality by design (QbD).
The vast majority of biotherapeutics are parenteral drugs — many of...
When it comes to clinical and commercial manufacturing of therapeutic products, outsourcing is an integral part of the biopharmaceutical industry. In the 21st century, product sponsors are increasingly relying on expert contract assistance in process development and production of clinical and commercial materials. Many companies are reaching beyond their local and national borders to extend networks of partnerships into emerging markets, particularly in Asia (
1, 2
).
Most biologics are proteins, with monoclonal antibodies (MAbs) dominating the scene over a number of other classes such as cytokines, blood factors, enzymes, and hormones. Vaccines and immunotherapies form a healthy segment as well. New product categories are growing fast: especially biosimilars, antibody–drug conjugates (ADCs), and cell therapies. And many experts project a growing need for outsourcing services in all these areas.
Contract manufacturing is not simply a cost-cutting measure (
3
). In many cases, it provides a means for produ...