May 2019 Featured Report
An antibody–drug conjugate (ADC) for cancer treatment needs four things to succeed clinically: It needs to target the right antigen using the right antibody, with the most powerful cytotoxin attached by best linker option. These features combine to endow the resulting conjugated product with therapeutic safety and efficacy.
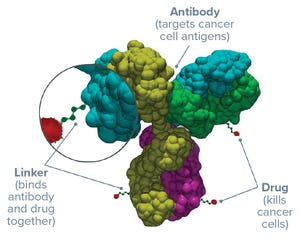
Figure 1:
Three parts of an antibody–drug conjugate
Antigens:
Determining which receptor to target is the first step in ADC development. The best options are expressed highly and consistently on the surface of a number of tumor cell types — but absent from normal tissues (or at least expressed at very low levels) — and won’t be downregulated after initial ADC treatment. If tumor cells stop expressing that antigen in reaction to their first exposure to a drug, then subsequent treatments won’t have the same cytotoxic effect. Tumor-specific antigens are the ideal choice, although so far most ADCs have targeted antigens that are only tumor “associated” (e.g., HER2 in breast cancer).
Anti...
The following news items have appeared on the
BioProcess Insider
site over the past year. Together they indicate the direction in which the ADC sector is moving.
(HTTPS://STOCK.ADOBE.COM)
BEYOND ADCETRIS — SEATTLE GENETICS AIMS FOR BIG BIOPHARMA STATUS
30 April 2018:
Seattle Genetics is building toward a bigger future. For the first quarter 2018, the company’s total revenues grew to US$141 million (€116 million) compared with $109 million in the same period last year. This was attributed to a 36% increase in sales of the Adcetris (brentuximab vedotin) ADC, which pulled in $95 million. With increased R&D expenses and acquisition of Cascadian Therapeutics, total costs for the quarter jumped by almost 30% to $234 million, so the company ended with a net loss of $112 million.
Progress Over Profits… for Now:
Profitability will come, said chief executive officer Clay Siegall. “We want to be profitable, we expect to be profitable, and we certainly could be profitable now based on our revenue if we skinny dow...
Speed to clinic testing — and then speed to market — are highly significant metrics for companies developing biopharmaceuticals. By increasing the pace of drug development, these companies can reduce costs, obtain revenues early, and establish commanding positions in the market relative to their competitors. High-throughput development tools have contributed much to the acceleration of drug development in recent years. Such technologies enable the testing of many process parameters in parallel. Combining them with multifactorial “design of experiment” (DoE) analysis leads to design of highly efficient and well-characterized bioprocesses.
One challenge that arises when companies adopt such an approach is that the manufacturing “bottleneck” shifts from process development groups to analytical testing laboratories. Small biotechnology companies often face a dilemma with respect to analysis of samples from early stage experiments: Either they must purchase expensive, sophisticated analytical technologies and ...
schedl_b_and_w.jpg?width=100&auto=webp&quality=80&disable=upscale)