November 2016 Supplement
Photo 1: GEMÜ Sumondo automation-capable single-use diaphragm valve meets purity requirements of USP and USP .
Single-use components and systems now are firmly established in the pharmaceutical and biotechnology industries. The trend toward simplified and flexible upstream and downstream plant design means that these components are becoming increasingly important — especially in biopharmaceutical production. In the past, the only available disposables were primarily tubes, fittings, and possibly filters. But the number of single-use systems has been increasing for a number of years now. It is hardly surprising that plant designers and operators now can rely on a large number of additional single-use components such as bioreactors, sensors, and pumps. Companies can implement a complete upstream process with a single-use design.
Automation capabilities have been limited because single-use systems did not have the full range of functions as their counterparts from traditional plant engineering. In 2014 GEMÜ ...
Figure 1: Sterilizing filter systems
Single-use components and systems have been incorporated into many bioprocesses as an alternative to cleanable, reusable systems. A wide range of publications have detailed the reasons for this trend toward a single-use approach. Justification in many cases comes from process-specific benefits such as increased manufacturing flexibility — especially for contract manufacturing organizations (CMOs) — enhanced sterility assurance, elimination of cleaning, reduced capital investment, faster processing times with increased productivity, faster start-up, and other benefits (
1
).
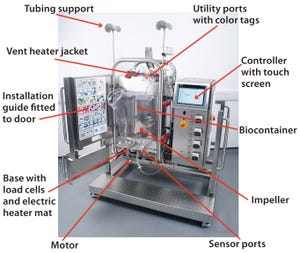
One critical factor in the application of single-use technology for biopharmaceutical manufacturing has been a need to design and develop components that fully satisfy the performance, functionality, and validation requirements for user process systems. Meeting this requirement is particularly difficult for complex equipment such as bioreactors (
1
). Here, we discuss how the challenge can be addres...
Special Report on Process- and Product-Related Impurities (A CMC Strategy Forum Special Focus Series): Extractables, Leachables, Particles, and AggregatesSpecial Report on Process- and Product-Related Impurities (A CMC Strategy Forum Special Focus Series): Extractables, Leachables, Particles, and Aggregates
In the biopharmaceutical industry, continuous manufacturing is often cited as a method for increasing the productivity of bioprocesses (
1
). Compared with batch processing, it has the potential to enable production of more product within a smaller facility footprint — while improving product quality, particularly for sensitive and unstable molecules. Investigation into continuous methods is taking place for both upstream and downstream operations. For the full benefit of continuous processing to be realized, an argument has been made that cell culture, harvest, and product purification must be effectively integrated (
2
). That follows a trend within the industry to take an increasingly holistic view of bioprocesses and bioprocess platforms encompassing seed bioreactors through downstream processing to drug substance freezing and thawing.
Cell culture operations play a vital role in determining overall process productivity. The industry has made great progress over the past couple decades in achieving hi...
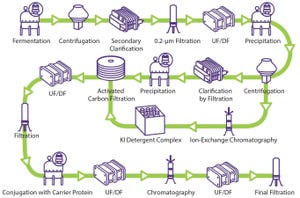
Figure 1: General polysaccharide conjugate vaccine process (UF/DF = ultrafiltration/diafiltration)
Polysaccharide vaccines are essential for protection against infectious diseases, which remain an alarming cause of mortality. The first glycoconjugate vaccine for use in humans — a
Haemophilus influenzae
type b (Hib) conjugate — was licensed in the United States in 1987. This vaccine successfully reduced the incidence of invasive Hib disease in childhood and led to the further development of conjugate vaccines designed to prevent infection by other encapsulated bacteria (
1
).
Table 1: General polysaccharide antigen properties
Polysaccharides are relatively complex carbohydrates made up of many monosaccharides joined together by glycosidic bonds. Bacterial polysaccharides represent a diverse range of macromolecules that include peptidoglycans, lipopolysaccharides, capsules, and exopolysaccharides — compounds whose functions range from structural cell-wall components (e.g., peptidoglycan) to important viru...