HTTPS://STOCK.ADOBE.CO
From transport and holding of bulk drug substance to shipping, warehousing, tracking, and distribution of final packaged drug products, biopharmaceutical supply chain logistics can be described as an industry in itself. And that’s just one side of the story. Even though much of the work of establishing and maintaining supply chains might happen outside the manufacturing environment, all organizations that develop processes and final products depend on having the raw materials and available components and resources to do their work.
A biopharmaceutical company’s “upstream” supply chain can be diverse, including everything from plastic components and the bioreactors they are used in, to cells, buffers, filters, columns, media, and more. All supplies must be subject to qualified procurement practices as determined and overseen by myriad departments and project managers. Sponsors of marketed products must ensure that they have adequate supplies of a consistent quality of all equipment a...
WWW.ISTOCKPHOTO.COM
Advancements in cell therapy, biofabrication, and synthetic biology have driven the growth of the global regenerative medicine (RegenMed) industry in the past decade. The industry has developed innovative treatment options for patients with otherwise unmet medical needs (
1
). Human or animal cells or tissues are used as critical raw materials in cell therapy products that can replace, regenerate, or augment patients’ diseased, dysfunctional, or injured cells, tissues, or organs. These cells or tissues can be unmanipulated, or their biological characteristics can be altered ex vivo before administration of a final product to patients. Examples of such treatments range from traditional blood transfusions to recent approaches in autologous stem cell transplants to allogeneic engineered tissue substitutes (
2
).
The complexities associated with manufacturing these “living drugs” should not be underestimated. Each product comes with its own specific challenges and complexities for managing...

 +1
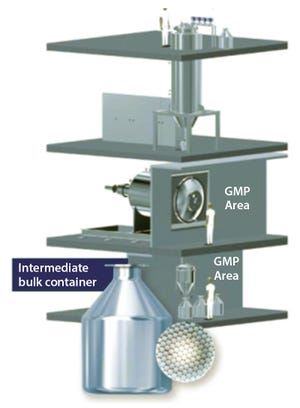
+1Figure 1: Production-scale equipment designed and manufactured by Meridion Technologies GmbH (
12
)
Biopharmaceutical drug substances (DSs) and drug products (DPs) commonly are stored frozen or refrigerated to maintain stability through long-term storage, handling, and transportation (
1
). Temperature excursions during storage and transport can affect product quality adversely by compromising the safety and efficacy of these molecules. Thus, cold-chain management throughout the shelf life of these products is a critical component in the supply chain strategy for them.
The cost and complexity of cold-chain management is a well-known challenge faced by the biopharmaceutical industry. Highly expensive and complex logistics can be attributed to several industry and regulatory-related factors, just a few of which are mentioned here. Adequate product storage equipment is required at all distribution nodes in the supply chain and must be qualified, maintained, and monitored continuously. Temperature-controlled ...

 +2
+2