November-December 2019 Featured Report
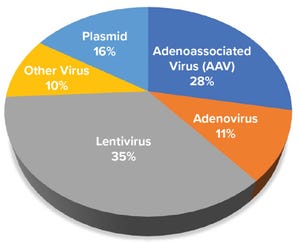
Figure 1: Distribution of gene-based advanced therapy medicinal products in development based on viral or plasmid platform used to express gene of interest
Advanced therapy medicinal products (ATMPs) are engineered to replace defective, disease-causing genes to compensate directly for a genetic defect or to encode a therapeutic protein construct (e.g., chimeric antigen receptor, CAR) for disease treatment. In most instances, a viral vector delivers the engineered genetic payload, targeting cells in situ or ex vivo through cellular modification, expansion, and infusion into a patient. Clinical successes of ATMPs bolstered by regulatory approval of products such as Luxturna (voretigene neparvovec-rzyl, Spark Therapeutics), Kymriah (tisagenlecleucel, Novartis), and Yescarta (axicabtagene ciloleucel, Kite, a Gilead Company) have dramatically increased funding and new-company formation. According to the Alliance for Regenerative Medicine (ARM), more than 700 active gene and gene-modified cell therapy clinical ...
Assay lifecycle development for traditional biopharmaceuticals such as vaccines and monoclonal antibodies (MAbs) has a clearly defined pathway, from preclinical method selection, development, and optimization through the milestones in preclinical phase trials, and finally to postlicensure method evaluations, comparability, and improvements. The analytical development roadmap for nontraditional biologics such as chimeric antigen receptor (CAR) T-cell therapies and gene therapies are not as clearly defined and can present many challenges along the way. Understanding the “what, how, and when” of analytical development for CAR T-cell products can help developers of such therapies find solutions to those problems. Andrea R. Moore, director of analytical development at Tmunity Therapeutics presented on the analytical testing strategies for these products at the BioProduction Congress in June 2019. This article is based on my discussion with her about that presentation.
Figure 1: Traditional assay lifecycle deve...
Cell and gene therapies (CGTs) are a novel and fast-growing class of transformative therapies designed to address gaps in traditional treatment strategies of some of the most severe diseases. By definition, gene therapy “seeks to modify or manipulate expression of a gene to alter the biological properties of living cells for therapeutic use”
(1)
. That can be either an in vivo delivery of a gene or delivery of a gene to a patient’s cells that are manipulated outside of the body and then redelivered, which is then considered a cell-based gene therapy.
In recent years, several CGTs have been approved for market. Such products include Kymriah (tisagenlecleucel, Novartis) and Yescarta (axicabtagene ciloleucel, Kite/Gilead), which are autologous T cells engineered to express chimeric antigen receptors (CARs) directed against CD19 for treatment of B-cell lymphoma; Luxturna (voretigene neparvovec-rzyl, Spark Therapeutics), which is an adenoassociated-virus–based gene therapy for the correction of RPE65 in treat...
Figure 1: Ideal AAV gene therapy development timeline
Adenoassociated virus (AAV) vectors have emerged as the prominent delivery mechanisms of corrective gene therapies. Three such products — Glybera (alipogene tiparvovec, uniQure), Luxturna (voretigene neparvovec-rzyl, Spark Therapeutics), and Zolgensma (onasemnogene abeparvovec-xioi, AveXis) — have been licensed, and a growing number of candidates are entering late-stage development. In mapping out an AAV gene therapy product development strategy, biomanufacturers should address fundamental considerations for their manufacturing strategies for both phase 1–2 clinical evaluation and translation for commercial market supply. A manufacturing strategy encompasses a supply chain of critical raw materials, a cell-substrate manufacturing platform, and a decision either to outsource manufacturing to contract development and manufacturing organizations (CDMOs) or to establish internal manufacturing capabilities. Herein we provide a snapshot of some key options a...