Content Spotlight
Podcast: MilliporeSigma says education vital to creating unbreakable chain for sustainability
MilliporeSigma discusses the importance of people, education, and the benefits of embracing discomfort to bolster sustainability efforts.

The ability to use AAVs for targeted therapeutic payload delivery has dramatically accelerated the development of life-saving gene therapies.
October 17, 2022

Sponsored Content
Characterize adeno-associated viruses (AAVs) for aggregation, stability, and particles at low volume for accelerated development of life-saving gene therapies.
Development can further be accelerated by monitoring critical quality attribute parameters like subvisible particles (SVP) concentration early in the development process. Capsid degradation and nucleic acid leakage can both occur under different formulation and storage conditions and contribute to the accumulation of aggregates. Characterizing candidates earlier in the development process is best as it identifies and eliminates inherently unstable candidates before they progress too far down the development workflow, saving significant time and effort.
However, producing AAV material is an expensive, time-consuming, and laborious process. Thus, AAV availability is a limiting factor when performing the experiments necessary to ensure the efficacy, stability, and safety of gene therapeutics in development. Gene therapy developers simply can’t spare the milliliters of material required by standard methods like flow microscopy or light obscuration to analyze subvisible particle formation or interrogate for DNA content, especially in earlier stages of development when there are many candidates to screen.
Aura® GT from Halo Labs overcomes this challenge as it only requires 5 µL of precious sample per analysis. You can now analyze replicates measurements when assessing AAV stability and monitoring potential DNA leakage, and still have enough material for additional characterization work. Particles are imaged on a membrane that captures particulates as samples are filtered through and imaged on Aura® GT using Backgrounded Membrane Imaging (BMI) technology. This approach increases the refractive index contrast 10x compared to standard methods, revealing translucent particles that can otherwise be missed. Aura® GT also uses particle-specific fluorescent dyes and fluorophore conjugated antibodies with Fluorescence Membrane Microscopy (FMM) technology to error-proof your particle ID. All you have to do is label particles in your sample with the dye either in solution or on the membrane and then image the membrane using one of three fluorescent channels. One sample can be labeled with multiple dyes, allowing you to definitively ID up to three particle types with FMM – all with just 5 µL of sample!
To demonstrate the ability to monitor DNA leakage, purified AAV was separated into empty and full capsid populations before they were stressed at 30 ˚C for one week to induce nucleic acid leakage. The samples were then applied to an Aura 96-well black membrane plate before staining with SYBR® Gold at 10x staining concentration for 5 minutes. The membrane plate was imaged on an Aura system to detect and quantitate SYBR Gold labeled DNA in subvisible AAV aggregates.
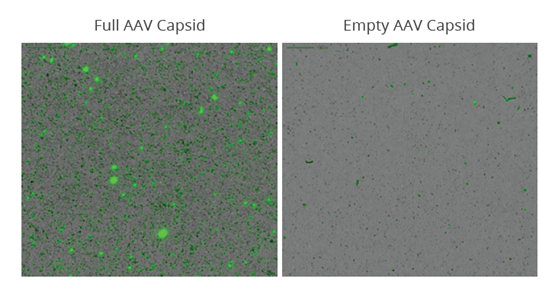
A full 96-well plate can be imaged in just a few hours, decreasing candidate screening time from a few weeks to a few days. Data analysis is automated with Particle Vue software, developing quantitative size, count, morphology, and ID information for every particle in your sample. For this sample, 16-fold more aggregation was observed in the stressed full AAV sample compared to the empty AAV sample.
The ultralow volume required of Aura® GT now makes it possible to assess the developability of potential gene therapeutics in different AAV serotypes early in the development process. With just 5 µL of sample, you can characterize and quantitate SVPs in your sample and determine the exact source of said SVP using FMM. Realize the promise of your gene therapeutic with Aura® GT!
Find out more here.
You May Also Like