- Sponsored Content
- Downstream Processing
Accelerating mRNA-Based Therapy Development with Scalable Purification of In Vitro Transcribed mRNA
September 25, 2020
Date: Sep 25, 2020
Duration: 20 Min
Sponsored by Thermo Fisher Scientific
This webcast features: Kelly Flook, Senior Product Manager, Purification Products, Thermo Fisher Scientific
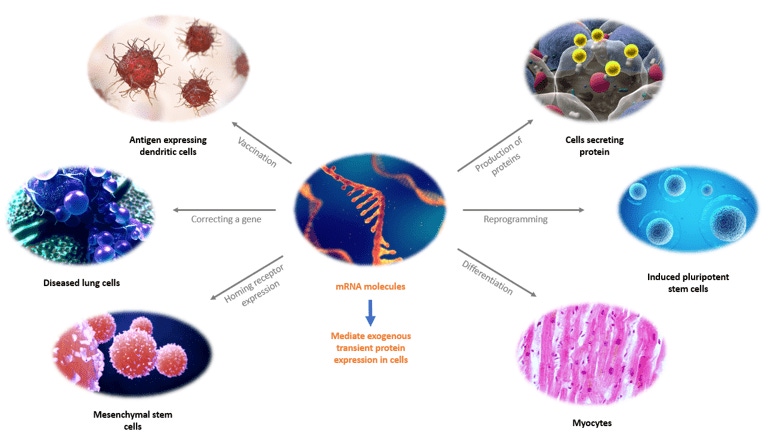
The diversity of potential mRNA-based therapies has led to increased interest in using synthetic mRNA as a tool in the treatment of multiple diseases, such as cancer, stem cell therapies, and infectious diseases. Nevertheless, obtaining larger quantities of synthetic mRNA for clinical treatment remains a challenge. Currently available mRNA purification methods are becoming a bottleneck for large-scale manufacture as the limits of research-scale purification techniques are realized. Affinity chromatography, a highly scalable method, has earned its credits in the development of biologics such as the use of Protein A for the purification of therapeutic antibodies and more recently anti-AAV resins in gene therapy workflows. An effective affinity purification step can help to simplify biomolecule downstream processing, reduce the number of purification steps, and reduce the overall cost of goods in biotherapeutic manufacturing.
Here we present our new affinity-based mRNA chromatography resin, specifically developed for the purification and isolation of mRNA from in vitro transcription (IVT) manufacturing processes.
Learning points:
Learn more about the new Thermo Scientific™ POROS™ Oligo (dT)25 affinity chromatography resin for the purification of IVT mRNA.
Understand how affinity chromatography can help to simplify workflows and maximize efficiency of your mRNA purification process.
Watch the recorded webcast now.
About the Author(s)
You May Also Like





