- Sponsored Content
- Gene Therapies
Addressing Challenges in Analyzing Heterogeneous AAV Populations
Sponsored by Refeyn
Adenoassociated virus (AAV) vectors are made from nonenveloped virus capsids that contain single-stranded DNA. As a leading delivery system for gene therapy, AAVs are in
development to treat a number of genetic diseases (1). As the industry has advanced, the number of clinical trials involving such vectors has risen dramatically (2), increasing the need for effective manufacturing and quality control (QC) methods.
During biomanufacturing, expressed AAV capsids can incorporate both target and host-cell DNA in a heterogeneous population. Viral capsids can be empty, partially filled, correctly filled, or overfilled. The desired capsids contain a full-length transgene needed for gene therapy.
Empty, partially filled, and overfilled AAV capsids are considered to be impurities and pose risks such as competing with functional, correctly filled vectors for host-cell attachment and entry, altering drug potency, and increasing the potential for immune reactions (3). Characterizing the ratio of correctly filled to empty, partially filled, and overfilled particles is thus essential for proper dosing, patient safety, and optimization of culture productivity and manufacturing consistency.
Optimization of AAV production depends on a developer’s ability to characterize rapidly such critical quality attributes (CQAs) as virus titer, capsid content, and aggregation. But the industry has lacked analytical capability for such rapid and accurate assessments. The analytical techniques most commonly used to characterize AAV preparations can require large amounts of material and time. The industry needs a more rapid, straightforward, and accurate way to meet the expanding demands of gene therapies.
Technologies for AAV Analysis
The current “gold standards” for detection and quantification of empty/full AAV capsid ratios are analytical ultracentrifugation (AUC) and transmission electron microscopy
(TEM). Other approaches include combining an enzyme-linked immunosorbent assay (ELISA) with quantitative polymerase chain reaction (qPCR) analysis. Here, we introduce an approach based on mass photometry that provides comparable results at the single-particle level, with lower sample demands and faster assay times than those options.
Characterizing AAV Capsids:
Cryogenic-TEM (cryo-TEM) is a type of electron microscopy in which samples are examined at liquid-nitrogen temperatures. This shows the structure and internal organization of AAV capsids in their native state, displaying a distinct difference between filled and empty capsids. This method requires high AAV concentrations, however, so that thousands of capsids can be imaged and analyzed for statistically significant results. Because TEM is a complex process requiring highly specialized equipment, it often is outsourced, making it a costly and timeconsuming choice.
Sedimentation-velocity analytical ultracentrifugation (SV-AUC) provides high resolution separation of viral populations based on their differences in density and mass. It is the de facto standard for quantifying heterogeneous capsid populations. A single AUC experiment provides insights into the masses of recombinant AAVs, the success of purification, and the presence of aggregates. However, AUC also can be slow, and it requires large sample volumes and extensive training to perform.
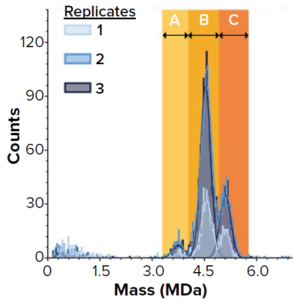
Figure 1: SamuxMP mass photometry measurements of heterogeneously filled adenoassociated virus (AAV) capsids at Pharmaron Gene Therapy; histograms show three distinct peaks believed to correspond to empty capsids (a) and two peaks of heterogeneously filled capsids (b and c). Three repeats were drawn from the same sample (technical replicates).
Mass photometry measures mass distributions of biomolecules and viral particles in solution with high resolution and sensitivity. Because it measures each particle individually, this technique provides information on sample heterogeneity and, as Refeyn data have shown, the presence of aggregates. Relatively small sample volumes (at a lower concentration than for AUC) are sufficient, and this method requires no sample preparation, so data can be obtained and evaluated in minutes. The Refeyn SamuxMP mass photometer is optimized for AAV analysis, providing results that are comparable to those of cryo-TEM and SV-AUC (Figure 1).
ELISA–qPCR is a well-established, sensitive, and specific method to quantify capsid and genome titers for determination of empty/full AAV capsid ratios. However, it is serotype dependent and can be too laborious and time-consuming for routine use (4). Additionally, the method provides limited accuracy and reproducibility because of the combined variability of ELISAs and qPCR.
Comparing Technologies
Sample throughput can be a concern in characterization of heterogeneous AAV populations during mass production of AAV vectors for gene therapies. SamuxMP mass photometry technology has been tailored to address that. Compared with cryo-TEM and SV-AUC, this system
• requires minimal training and prior knowledge for benchtop analysis
• works with just 10–20 μL of sample at a low concentration (1011 particles/mL) so that far less material is lost to analysis
• processes samples in minutes rather than hours, better facilitating effective manufacturing.
Those attributes make Refeyn’s SamuxMP system an ideal candidate for processing AAV capsids in an industrial context.
Pharmaron Gene Therapy, a contract development and manufacturing organization (CDMO), evaluated the technology’s speed and robustness for processing small volume samples. First, the team tested whether the SamuxMP could differentiate between different populations of capsids in a heterogeneous sample. Each technical replicate of a commercially available AAV standard showed three distinct peaks (a, b, and c) in the correct mass range for AAVs (Figure 1). Peak a corresponds to the known mass of empty AAV capsids; b and c are filled with increasing quantities of genetic
material.
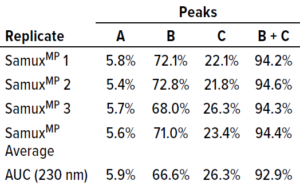
Table 1: Comparing SamuxMP mass photometry and analytical ultracentrifugation (AUC); averaged massphotometry measurements from three technical replicates of three distinct capsid populations in one sample (Figure 1) agree well with AUC measurements of the same sample. All data were provided by Pharmaron Gene Therapy.
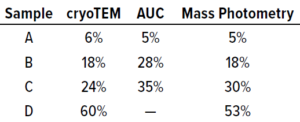
Table 2: Benchmarking cryogenic transmission electron microscopy (cryo- TEM), AUC, and SamuxMP mass photometry methods used to measure the percentage of full capsids in each of four adenoassociated virus (AAV) samples containing different proportions of empty and full capsids; data were provided by the Cell and Gene Therapy Catapult.
Quantification results showed consistency among measured proportions of capsids in each population across all three technical repeats (Table 1). AUC analytical results were equivalent. Thus, the SamuxMP instrument can distinguish and quantify heterogeneous populations in capsid samples both reliably and precisely to an extent that is comparable to the current gold standard of AUC, but requiring less sample and time. Those results complement findings from a previous investigation by the Cell and Gene Therapy Catapult, which used cryo-TEM, AUC, and SamuxMP analysis to determine the empty/full ratio of AAV capsids in different samples. That study demonstrated similar results across all techniques (Table 2).
An Attractive Option
AAVs are a rapidly growing and potent new class of biopharmaceutical vector requiring improved analytical capabilities for rapid and accurate assessment of sample heterogeneity at increasing throughput. Commonly used methods for characterizing AAV preparations are too slow for use in mass production, can have poor accuracy, and require large sample amounts and/or preparation. Mass photometry offers an alternative. The SamuxMP system can obtain accurate data quickly, with minimal sample consumption. It has been demonstrated to deliver robust results that are comparable to those of goldstandard technologies. Those attributes, combined with ease of use and a small benchtop footprint, make mass photometry attractive for rapid characterization of viral vectors in both industrial and academic applications.
References
1 Mendell JR, et al. Current Clinical Applications of In Vivo Gene Therapy with AAVs. Mol. Ther. 29(2) 2021: 464–488; https://doi.org/10.1016/j.ymthe.2020.12.007.
2 Kuzmin DA, et al. The Clinical Landscape for AAV Gene Therapies. Nat. Rev. Drug Disc. 20(3) 2021: 173–174; https://doi.org/10.1038/d41573-021-00017-7.
3 Verdera HC, Kuranda K, Mingozzi F. AAV Vector Immunogenicity in Humans: A Long Journey to Successful Gene Transfer. Mol. Ther. 28(3) 2020: 723–746; https://doi.org/10.1016/j.ymthe.2019.12.010.
4 Gimpel AL, et al. Analytical Methods for Process and Product Characterization of Recombinant Adeno-Associated Virus-Based Gene Therapies. Mol. Ther. Met. Clin. Devel. 20, 2021: 740–754; https://doi.org/10.1016/j.omtm.2021.02.010.
Svea Cheeseman ([email protected]) is director of product management for cell and gene therapy at Refeyn Ltd (www.refeyn.com). Paul Getty is a senior technical specialist in analytical sciences at Pharmaron Gene Therapy. Kirsty McManus is a senior scientist in the characterization team at Pharmaron Gene Therapy.
You May Also Like





