Quantitation of different capsid species in viral-vector gene-delivery drugs, including recombinant adenoassociated virus (AAV) therapies, is essential for proper assessment of critical quality attributes before regulatory approval and during commercial production. If rAAV full-capsid percentages are maximized, and variants such as empty or partially-filled capsids are reduced as much as possible, then potency is increased for improved dosing and tolerance outcomes.
Traditionally, characterization of AAV purity with respect to empty, full, and other variants has been performed by techniques such as electron microscopy. Separate protein/nucleic-acid quantitation has been performed by dual assays such as enzyme-linked immunosorbent assay (ELISA) and quantitative polymerase chain reaction (qPCR). Such approaches have some shortcomings, however. For example, capsid and genome quantification by ELISA–qPCR or UV absorbance suffers from poor accuracy, and methods such as high-performance liquid chromatography (HPLC) and capillary electrophoresis (CE) have not yet achieved the separation required to quantify discrete populations of empty, full, and partial capsid species reliably.
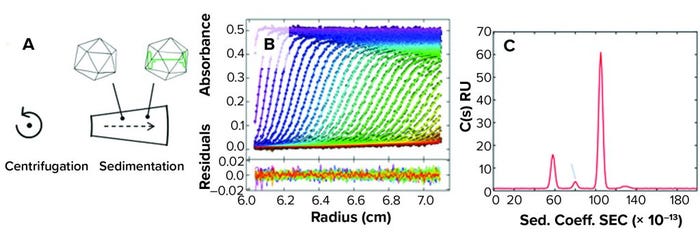
Figure 1: (A) Analytical ultracentrifugation (AUC) uses centrifugal force to separate species based largely on mass differences, and (B) raw data measurements are taken in real time. (C) Sedimentation data are deconvolved into a size-distribution profile reflecting the relative size and abundance of the separated species. (Sed. Coeff. = sedimentation coefficient, C(s) = sedimentation coefficient distribution, RU = resonance unit).
This article describes how assays using analytical ultracentrifugation (AUC) can quantitate empty, full, and other capsids for reliable characterization of AAV purity. Fill out the form below to read the complete article now.








