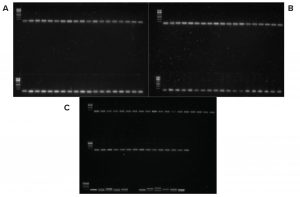
Figure 1: PCR products gel electrophoresis; no viral nucleic acid was detected. The first spot on each panel (left corner) is the DNA ladder, and the bottom row on C shows the positive controls. Left to right are BTV, BCV, PRRSV, CSFV, PCV2, BP, BPV, PPV, ASFV, SVDV, and BHV. Smallest fragments in every spot are primer dimers.
Serum is the most commonly used supplement in cell culture. Fetal bovine serum (FBS) is the common choice because it contains high concentrations of growth factors and other important signaling molecules (e.g., adhesion proteins, nutrients, carrier proteins, cytokines, and hormones) required for cell survival and differentiation together with its buffering capabilities.
FBS production begins with the collection of whole blood from bovine fetuses under aseptic conditions. Once collected, the blood is allowed to clot, and the serum is mechanically separated. Raw serum is kept under sterile conditions and low temperature. Further processing such as filtration and irradiation typically are applied to eliminate undesired components and inactivate viruses. Thus, animal health and slaughter conditions must be highly controlled and meet strict sanitary specifications.
Initially, endotoxin and hemoglobin content are measured, and approved FBS batches might undergo extra quality control (QC) tests to determine compliance with quality assurance (QA) requirements. This process usually includes physical and chemical tests, biochemical assays, microbiological tests, and cell-culturing assays when growth experiments are applied to test whether FBS batches can support cell growth and demonstrate suitability for different cell lines.
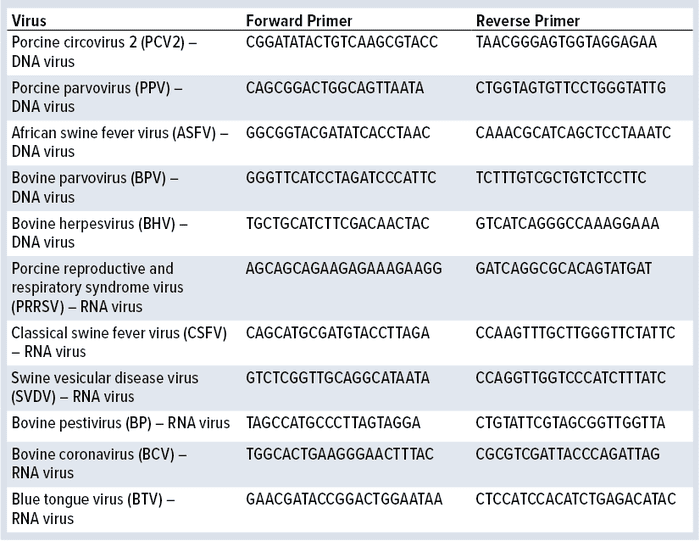
Table 1: Viruses and primer sequences used in this study
FBS origin and widespread assumptions about countries having a few cases of cattle disease are major concerns among users. Related assumptions stem from reported cases of bovine spongiform encephalopathy (BSE) outbreaks. Initially, countries with no reported cases were regarded as safe options. Later, government and regulatory agencies sided with scientific data that determined that BSE is not transmitted in blood-derived products as long as proper slaughter conditions are met. However, viral presence still is a concern and addressed by regulations such as USDA 9 CFR 113.46–53 and EMEA-CPMP-BWP-1793-02. Those state that regardless of the country of origin, FBS must be tested and/or treated for the presence of contaminating viruses.
The study herein directly compares JBS serum produced from federally inspected (Brazilian Ministry of Agriculture) and fully traceable cattle against certified and defined sera. Researchers accessed the presence of 11 viruses following 9 CFR procedures and tested cell growth using three different cell lines: monkey kidney epithelial cells, engineered progenitor B cells, and mouse primary neural stem cells from the subventricular zone.
Materials and Methods
Cell Culturing: All three cell lines were maintained in media supplemented with 10% (v/v) FBS, penicillin (100 U/mL), and streptomycin (100 μg/mL) (Gibco, CA) at 37 °C in a humidified atmosphere of 5% CO2. At 80–85% confluence, cells were detached (when applicable) with a solution of pancreatin (2.5%) diluted 1:10 (v/v) in Earle’s balanced salt solution (EBSS), collected by centrifugation, and suspended in media as described above.
Stem cells were cultured in specific media supplemented with growth factors and cell-differentiation inhibitors. Engineered progenitor B cells are nonadherent, so they do not require detachment for long-term culturing.
9 CFR Assay: Monkey kidney epithelial cells were cultured as described above for 21 days with the different FBS samples tested. After that period, cells were harvested. Nucleic acid (DNA and RNA) was extracted using TRIzol reagent (Thermo Fisher Scientific) and further tested for the presence of viral DNA and RNA after polymerase chain reaction (PCR) and reverse transcriptase PCR (RT-PCR) (SuperScript IV, Thermo Fisher Scientific) followed by PCR reaction, respectively. Table 1 shows primers used for nucleic acid amplification. Primer specificity was confirmed with vectors containing their target sequences. PCR products were separated in agarose (1%) electrophoresis gel stained with SYBR Safe DNA gel stain (Thermo Fisher Scientific) and visualized under ultraviolet light.
Proliferation assays were performed using the MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-bromo diphenyltetrazolium) method. Cells were plated in 96-well plates and cultured with the different FBS samples. After 24–72 hours, researchers added 350 μL to each well of MTT solution (5 mg/mL). After a four-hour incubation, cell supernatant was removed, and 500 μL of dimethylsulfoxide (DMSO) were added to all wells to lyse cells and solubilize crystals. Absorbance was determined at 570 nm. Use of MTS (3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium) provided direct measurement, and absorbance in this case was measured at 490 nm.
Results
9 CFR Assay: Monkey kidney epithelial cells were cultured for 21 days in supplemented media containing 10% of the different FBS samples. After that period, cells were harvested and nucleic acid was extracted. PCR reactions assessed whether cells were infected by any of the 11 viruses listed in Table 1. For RNA viruses, total RNA was converted into cDNA using reverse transcriptase followed by PCR amplification with gene-specific primers. In total, 10 batches (four control FBS batches) were studied.
Figure 1 shows gel electrophoresis of the PCR reactions in which no viral nucleic acids were detected. Researchers assessed primer specificity using designed vectors containing their target sequences.
Results from the 9 CFR assay show that no FBS batches contained active viruses. Results had no correlation with their origins and production process stages. FBS sourced from the United Stated, Australia, New Zealand, and Brazil (JBS) all were free of active viruses.
Proliferation Assay: Monkey Kidney Epithelial Cells: Researchers cultured monkey kidney epithelial cells and conducted proliferation assays as described above. Cells were plated in 96-well plates and cultured in media supplemented with 10% FBS of different origins for 48 hours. Researchers added 350 μL of MTT solution (5 mg/mL) per well. After a four-hour incubation, cell supernatant was removed, and 500 μL of dimethylsulfoxide (DMSO) were added to all wells to lyse the cells and solubilize the crystals. Absorbance was measured at 570 nm.
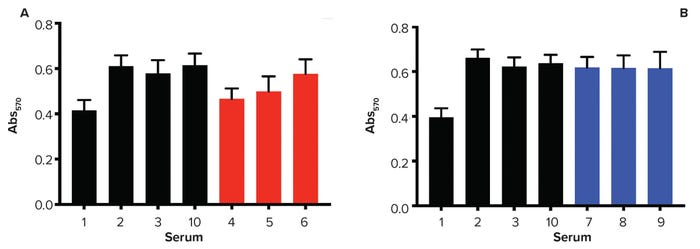
Figure 2: Monkey kidney epithelial cell proliferation assay; cells were cultured for 48 hours before exposure to MTT solution. Higher values of Abs570 indicate greater numbers of metabolically active cells. FBS is sourced from 1 = New Zealand, 2 = United States, 3 = Australia; 10 = control; 4–6 = finished products (filtered, red); 7–9 = raw sera (blue).
Figure 2 lists results from all FBS samples. Out of 10 FBS tested, the New Zealand-sourced FBS (number 1 in the figure) presented the lowest proliferation capability. Among the JBS samples (numbers 4–6) are finished products (filtered, red), and raw sera are shown in blue (numbers 7–9). Samples 4 and 5 presented a lower proliferation capability when compared with Sample 6, but not as low as the FBS sourced from New Zealand. However, all raw samples showed similar results to the controls (number 2 is US-sourced and number 3 is Australia-sourced FBS). FBS number 10 was added as a control because it is the FBS commonly used at the testing-site laboratories (Parker Haworth Bioscience).
Monkey kidney epithelial cells also were cultured for 21 days according to 9 CFR assay methods, which allow long-term monitoring. Pictures were taken every three to four days, and all of the sera but numbers 4 and 5 supported cell growth and survival throughout. Regardless of the cells’ origin and production-process stage (finished or raw), their growth and survival were supported.
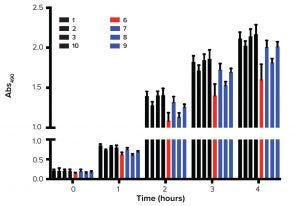
Figure 3: Growth kinetics; greater values of Abs490 represent greater numbers of metabolically active cells. FBS is sourced from 1 = New Zealand, 2 = United States,
3 = Australia; 10 = control; 6 = finished product (filtered, red); 7–9 = raw sera (blue).
Researchers also used MTS assay because it allows direct measurement of cell growth. Batch numbers 4 and 5 were excluded from this experiment because of problems observed in long-term cultures. Figure 3 shows the growth profile over a four-hour period. Again, control and JBS sera presented similar growth profiles with no significant differences between finished and raw samples.
Collectively, data show that FBS samples of different origins and even at different production stages support growth. In the short-term culture and growth profile, no significant differences were detected. Differences were observed after 48 hours of culturing (New Zealand-sourced) and long-term culturing (JBS 4 and 5).
Engineered Progenitor B Cells: Researchers also studied cell proliferation in a nonadherent cell model. The engineered progenitor B cells grow in suspension and do not express canonical growth factors or receptors (FGF and FGFR). Together with FBS, IL-3 is required for cell proliferation in this model.
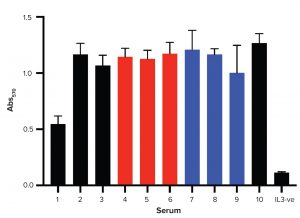
Figure 4: Progenitor B cells proliferation assay; cells were cultured for 24 hours before exposure to MTT. Higher values of Abs570 mean greater numbers of metabolically active cells. IL3-ve is the negative control in which no IL-3 was added.
Figure 4 shows results from all FBS samples. For the monkey kidney epithelial cell proliferation assay, FBS 1 (New Zealand-sourced) presented the lowest proliferation capability. All JBS batches — regardless of production- process stage — supported cell growth. No significant difference is observed among JBS samples and controls 2 and 3. JBS samples 4 and 5 presented similar results to other samples. That suggests that the lower proliferation observed for monkey kidney epithelial cells might be related to adhesion proteins, which is irrelevant in this cell model. Together, the data show that FBS samples of different origins and at different production stages support growth of progenitor B cells.
Mouse Primary Neural Stem Cells: Stem cells can develop into specialized cell types in the host body. They divide in self-renewal to produce more of the same type of stem cells. Different molecules are known to support stem-cell growth and differentiation, and FBS is an important source of such molecules. Researchers tested cell growth in mouse primary neural stem cells after 48 hours. JBS sera and control samples were tested.
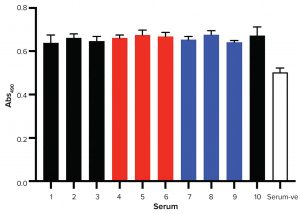
Figure 5: Mouse primary neural stem cells proliferation assay; greater values of Abs490 represent greater numbers of metabolically active cells. Serum-ve is the negative control with no serum added to the growth media.
Figure 5 shows results of the MTS assay. Clearly, all FBS tested supported stem-cell growth equally. Regardless of their origin and production stage, all samples supported stem-cell growth. FBS also is a source of molecules that induce stem-cell differentiation. Cell differentiation typically is accompanied by changes in cell morphology. After 48 hours of culture, images were made for every condition (Figure 6). Cells went through significant morphological changes in all cases. With no serum exposure, cells were spherical with low spreading. After being exposed to the different sera, cell spreading was observed.
Summary and Possible Further Work
FBS samples sourced in the United States, New Zealand, Australia, and Brazil were tested for cell infectivity (using assay in 9 CFR) and cell proliferation using three different cell lines. Two adherent cell lines (monkey kidney epithelial cells and neural stem cells) and one nonadherent (progenitor B cells) were used.
Monkey kidney epithelial cells were cultured for 21 days and nucleic acid was extracted for the determination of possible cell infection by active viruses present in the different FBS samples. After PCR amplification using gene-specific primers for 11 viruses, no viral genetic material was detected. Despite being sourced from different countries (some of which are believed to be “safer” options), no FBS sample contained active viruses.

Figure 6: Mouse primary neural stem cells were grown in media containing penicillin (100 U/mL), streptomycin (100 µg/mL) (Gibco, CA) and growth factors. Cells were cultured at 37 °C in a humidified atmosphere of 5% CO2 in the absence (a) or presence (b) of FBS. Images were made before supplementation with FBS (a) and after 48 hours of culturing in media supplemented with a specific FBS (b). Note the morphological changes when FBS is present, suggesting that the FBS sample may have induced cell differentiation. (Slides A = ES cells, no FBS; slides B = ES cells, specific FBS as shown after 48 hours).
Cell proliferation also was tested in monkey kidney epithelial cells. Most FBS samples showed similar proliferation profiles. FBS sourced from New Zealand (number 1) and from JBS (numbers 4 and 5) decreased proliferation, but source 1 was unquestionably the worst. Long-term cultures also were assessed, and JBS 4 and 5 resulted in impaired long-term growth. Such findings might be related to decreased levels of adhesive proteins required for cell survival.
Using FBS sourced from New Zealand also led to weaker proliferation in progenitor B cells, but in that case, JBS 4 and 5 were similar to the other FBS samples. Because progenitor B cells are nonadherent, those results support the idea that decreased levels of adhesive proteins are present in JBS 4 and 5.
Finally, the different FBS samples were tested using mouse neural stem cells. Again, regardless of origin and production stage, all samples supported cell proliferation. Morphological changes were detected in all cases, which suggests that the FBS samples might have induced cell differentiation. Future work could be performed to determine whether the different FBS samples induced cell differentiation to different cell lineages.
Collectively, results presented in this study support the idea that there is no “safer” choice of FBS. Furthermore, because biological products were used in different biological models (different cell lines), sample variability is indeed expected.
Paulo A. Reimann is director of JBS Orygina, Av. Marginal Direita do Tietê, 500 São Paulo, Brazil CEP, 05118-100; 55-11-98162-0159; [email protected]; www.jbs.com.br.
Study conducted in concert with Parker Haworth Bioscience.














