Voices of Biotech
Podcast: MilliporeSigma says education vital to creating unbreakable chain for sustainability
MilliporeSigma discusses the importance of people, education, and the benefits of embracing discomfort to bolster sustainability efforts.
Sponsored by GE HealthCare Technologies
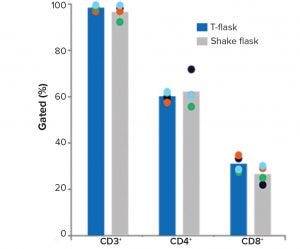
Figure 1: Comparison of small-scale culturing methods; phenotypic analysis of T cells and subpopulations CD4+ and CD8+ cells by flow cytometry at the end of culture; bars represent the average of four biological replicates with individual values shown by dots.
It is an exciting time to be involved in the cell and gene therapy industry. We have come to a tipping point where we have shown that the science works. Now we need to industrialize and standardize it, so we can get these life-saving therapies to more patients.
As an industry, we can learn from all the experience in the biologics space. With monoclonal antibodies (MAbs), we have transitioned from a manual process to a more standardized and automated set-up. We want to do the same thing for cell and gene therapies — but much faster.
We need to come to a standardized set-up and get to a closed and automated system. That will take us to a platform that will serve large patient populations while reducing both costs and risks. GE Healthcare Life Sciences has made good progress on this front for autologous chimeric antigen receptor (CAR) T-cell production, as described in the case study herein.
This article describes an automated CAR T-cell development process that uses commercially available reagents compatible with good manufacturing practice. Just fill out the form below to read it now.
Acknowledgments
All work was performed in collaboration with CCRM through funding from FedDev Ontario and GE Healthcare Life Sciences at the Centre for Advanced Therapeutic Cell Technologies (CATCT), Toronto, ON, Canada. The reporting and interpretation of the research findings are the responsibility of the authors.
Corresponding author Rohin Iyer is development manager, cell and gene therapy. Catarina Flyborg is general manager, cell and gene therapy at GE Healthcare Life Sciences.
You May Also Like