Voices of Biotech
Podcast: MilliporeSigma says education vital to creating unbreakable chain for sustainability
MilliporeSigma discusses the importance of people, education, and the benefits of embracing discomfort to bolster sustainability efforts.
Sponsored by Tosoh Bioscience
Tosoh Bioscience’s SkillPak 1-mL and 5-mL prepacked columns
Some of the latest promising biopharmaceutical drug substances are antibody fragments. Antibody fragments are either separate functional subunits of antibodies or recombinant molecules, which, just like antibodies, are composed of immunoglobulin domains. These drugs offer several therapeutic advantages over conventional monoclonal antibodies.
Upstream processing for antibody fragments is easier than it is for standard antibodies. Recombinant-based antibody fragments can be modified to meet specific needs of affinity, avidity, valence, and action mode. They also can be produced in prokaryotic cells (such as Escherichia coli), a cell line more economic than mammalian expression systems. Downstream processing, however, can be challenging. As antibody fragments do not have an Fc-region, chromatography experts cannot use protein A affinity and must select protein L as a ligand for fragment capture. Protein L is a surface protein of Peptostreptococcus magnus, which binds the κ-light-chain of antibodies present in most common fragments.
Tosoh Bioscience has developed Toyopearl AF-rProtein L-650F as the best-in-class affinity resin for antibody fragment capture. Our ligand is a genetically optimized recombinant protein L with high chemical stability. After linking that ligand to the well-established Toyopearl rigid methacrylic polymer backbone, we obtained a stable resin with high dynamic binding capacity (DBC) at fast flow rates. That improves the efficiency and reduces the cost of capture steps for antibody fragments.
 Method Development
Method Development
The goal of the protein L capture step is to reduce the levels of host-cell protein (HCP) and DNA contaminations while increasing concentrations of target fragments. We developed a process that allows those two main contaminants to flow through a column bed while target molecules are bound and later eluted from the column at low pH. Table 1 lists process conditions by which we achieved an HCP log reduction of 3.37, a DNA log reduction of 4.30, and a recovery of 95.3%.
Dynamic Binding Capacity
We compared the DBC for two different fragments at different flow rates using Toyopearl AF-rProtein L-650F resin and Capto L resin (from Cytiva) (Figure 1).
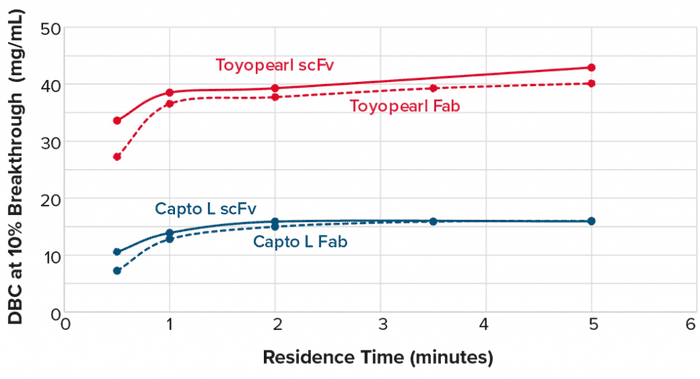
Figure 1: Toyopearl AF-rProtein L-650F resin exhibits a 2.5 times higher dynamic binding capacity (DBC) for scFv and Fab compared with Capto L resin. Equilibration buffer: 0.1 M sodium phosphate, pH 7.0, elution buffer: 0.1 M glycine/HCl, pH 2.5.
Compared with the DBC of Capto L resin, the DBC of Toyopearl AF-rProtein L-650F resin is roughly 2.5 times higher at any given residence time. From a process economics viewpoint, that means that a process using Toyopearl AF-rProtein L-650F will require 2.5 times less resin to purify the same amount of target fragment than a process using Capto L resin. Moreover, a higher DBC enables the use of highly concentrated elution pools, reducing the costs of subsequent purification steps.
Alkaline Stability
Toyopearl AF-rProtein L-650F resin shows a higher alkaline stability than Capto L resin. A typical NaOH-contact time of 15 minutes allows running 40 cycles when using the Toyopearl AF-rProtein L-650F before dropping below 90% SBC, but only 19 cycles when using Capto L resin. With that higher alkaline stability and higher DBC, a bioprocess that includes Toyopearl AF-rProtein L-650F resin will require five times less resin to purify an amount of target fragment when that same process uses Capto L resin.
Flow Characteristics
High DBC at fast flow rates increases process productivity. However, working at such flow rates is possible only if backpressure remains moderate. Toyopearl AF-rProtein L-650F resin exhibits a low pressure drop at all flow rates up to 1.000 cm/h. That increases flexibility and productivity for batch and continuous applications.
Product Recovery
Product recovery reaches 95.3% for the Toyopearl AF-rProtein L-650F resin and 92.7% for Capto L resin. That contributes to better economics of a capture step with Tosoh Bioscience’s protein L resin.
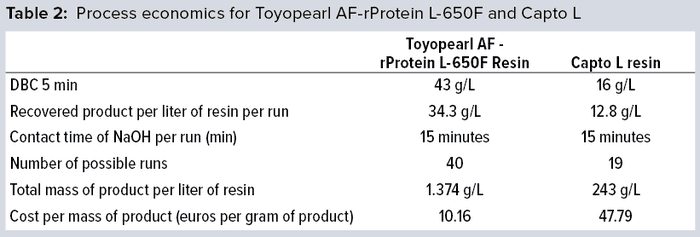
Overall Process Economics
Table 2 lists the process economics of a real-life capture-step. Running a fragment capture step on Toyopearl AF-rProtein L-650F resin costs only 21% of the cost of the same process using optimized conditions on Capto L resin.
The Resin of Choice for Antibody Fragment Capture
Toyopearl AF-rProtein L-650F resin combines high recovery, high DBC, high alkaline stability, and excellent flow characteristics. It can be packed easily in larger columns, enabling implementation in bioproduction processes. Toyopearl AF-rProtein L-650F resin needs to be repacked less often thanks to its high alkaline stability, thereby reducing costs for resin purchase and saving time for column packing and evaluation. A resin with high DBC combined with good elution characteristics enables highly concentrated elution pools, which cannot be achieved with low-DBC resins. High flow stability allows for high linear flowrates. In turn, that shortens process duration and increases process productivity. Toyopearl AF-rProtein L-650F resin reduces the costs of the capture step by almost 80% compared with Capto L resin.
If you want to impress yourself with the benefits of using Toyopearl AF-rProtein L-650F resin for the purification of your antibody fragments, inquire a complementary set of SkillPak columns or technical advice and support from Tosoh Bioscience’s chromatography experts. Go online at www.surveymonkey.de/r/AFrProteinL.
Jonas Wege is a technical specialist, and Romain Dabre, PhD, is a senior product manager at Tosoh Bioscience GmbH, Im Leuschnerpark 4, 64347 Griesheim, Germany; 49-6155-7043700; [email protected].
You May Also Like