Voices of Biotech
Podcast: MilliporeSigma says education vital to creating unbreakable chain for sustainability
MilliporeSigma discusses the importance of people, education, and the benefits of embracing discomfort to bolster sustainability efforts.
Cell culture medium is critical to cell growth, metabolism, and protein expression. It provides for optimum pH, osmolality, and nutrients in an environment that is essential for cell survival, growth, and expression of proteins and/or metabolites and drug-substance modalities of interest (1). A complete medium typically contains basic nutrients such as carbohydrates, amino acids, lipids, salts, vitamins, trace metals, growth factors/hormones (e.g., insulin), antishear factors, and other chemicals that facilitate cell growth and protein expression and may stabilize recombinant protein products secreted from cells. Many formulations currently used by biopharmaceutical companies are based on modifications to classical media compositions such as Dulbecco’s Modified Eagle’s Medium (DMEM) and Ham’s F12 media.
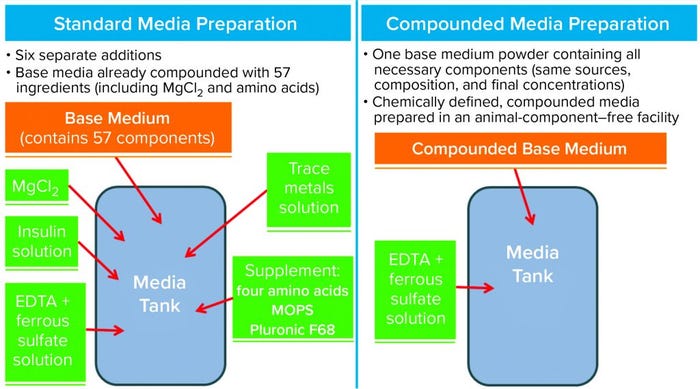
Figure 1: Standard medium preparation (left) and process using compounded medium (right); most (57) medium components are in the base media powder, with additional components added in house to complete the formulation. FeSO4–EDTA, a panel of trace metals, and rHu insulin are prepared as stock solutions and added as needed to media batches, whereas magnesium chloride and “supplement” are added as powders. Compounding of the FeSO4–EDTA was not feasible (see text for details). EDTA = ethylenediaminetetraacetic acid; MOPS = morpholinepropanesulfonic acid
When selecting cell culture media, taking a platform approach begins with the same basic formulation to support growth of similar cell types and lines producing different protein products. For meeting product quality attributes (PQAs) and productivity requirements best, “tailoring” a medium formulation (to both the cells and process being developed) is the current norm. That’s the case even when the same host cell line is used to produce similar products using similar processes and systems — e.g., perfusion-grown baby hamster kidney (BHK) cells expressing coagulation factors or fed-batch grown DG-44 Chinese hamster ovary (CHO) cells expressing monoclonal antibodies (2, 3).
Such media development efforts typically take place during early process development to help improve culture performance. High specific productivity, growth rates, densities, and secretion are examples of commonly desired cell attributes, along with phenotypic differentiation and genetic stability. Quality attributes depend on the type of product produced (e.g., an expressed protein or the cells themselves). Specific glycosylation profiles and high titers are common targets for expressed protein products. But medium preparation methods that result from a tailoring approach performed to tight development timelines can end up with a less-than-ideal batching process getting transferred into commercial operations.
Media Development and Batching
One common media tailoring/optimization approach leverages design of experiments (DoE) methodology, a statistical approach in which different components are combined at differing levels (as inputs) to identify an optimal formulation based on defined targets (outputs) (2, 3). Often a general, classic medium is used as a base, then key components (or groups of components) are left out as separate additives to enable high-throughput optimization experiments that can help developers identify the best possible composition.
DoE is a powerful strategy that can provide desired outputs while saving valuable development time. The approach often leverages a qualified and representative scaled-down cell culture model system to increase experimental throughput. That raises confidence that the medium composition that ultimately results will remain optimized after scale-up and through implementation at the commercial manufacturing scale (4).
Purchasing both the base media and additives (to be used in the DoE) from a reputable supplier offers many advantages (5). A base medium can be a single- or multicomponent premixed product in powder or liquid form purchased either as an off-the-shelf product or custom prepared by a supplier to customer specifications.
Batch Preparation: Under rising pressure (often driven by cost and/or market competition) to shorten development timelines, biopharmaceutical companies increasingly end up handing over a nonstreamlined process of media preparation from development to manufacturing groups. For example, whereas grouping components is a powerful DoE approach for media tailoring and optimization, adding individual (groups of) components during routine media batch preparation in commercial manufacturing is no longer an advantage — and in fact, it is inefficient. Such inefficiency is more pronounced when media are used in continuous production processes (e.g., perfusion) because of the large media volumes required.
Preparation of cell culture media is a complex process, especially in a commercial setting. It requires stocking, transfer, mixing, and storage of multiple stock solutions and powders — and even more when sequential batching is needed (Figure 1, left panel).
However, solid components sometimes are added and hydrated sequentially to prevent undesirable interactions among media components and facilitate dissolution to homogeneity (which is especially challenging for richer and more complex formulations). However, the larger the number of components and steps in preparation, the less efficient and more expensive a media-batching process will be. Multistep processes also increase the opportunity for introduction of variability, which in turn can affect cell growth, performance, and protein production consistency.
We reduced such complexity and inefficiencies significantly by augmenting or further compounding a base dry-medium powder with components that traditionally had been added separately during commercial media batching. We did not change the composition of our fully hydrated medium, only the preparation of dry medium powder and batching process. Below, we demonstrate the feasibility of compounding at three levels: first, through complete dissolution of dry-powdered medium; second, by verifying that the correct composition of fully hydrated liquid medium was maintained; and third, by showing that culture performance and recombinant protein production remained comparable, as did key tested PQAs.
Compounding Results
We discovered through experiments using dry-media components compounded to various extents (6) that it is feasible to compound base medium powder with the exception of a single component (Figure 1, right panel): ferrous sulphate with ethylenediaminetetraacetic acid (FeSO4–EDTA). Elemental analysis showed that including it at the dry-powder stage yielded about 30% lower levels than target (not shown) in the final formulation. Those empirical data suggest that FeSO4–EDTA does not dissolve properly when included in a compounded medium. Moreover, a brownish residue became apparent on the 0.22-µm membrane when we filtered a completely compounded medium (including FeSO4–EDTA), which indicated immediate oxidation of Fe(II) ions. Trace-element analysis also showed that the order in which components were added was important, probably because EDTA can cause undesirable chelation of other bivalent trace metals in a medium. Our results showed that all other components that previously had been added to media one at a time during in-house batching could be included in the (compounded) base medium powder.
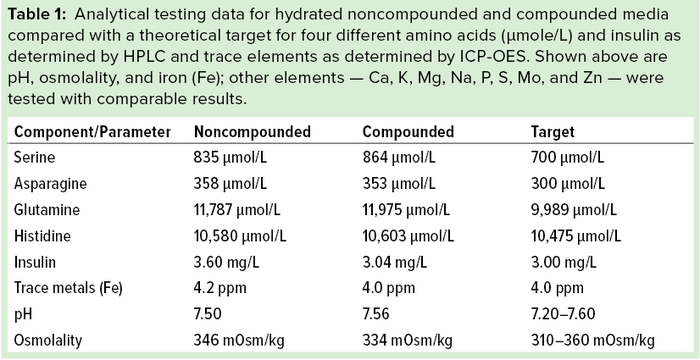
Hydration studies demonstrated feasibility of further compounding components in our dry-medium powder. We verified their concentrations in hydrated liquid media and benchmarked those against a target concentration to compare with noncompounded media. The near-complete dry-powder formulation (with the exception of FeSO4–EDTA) — referred to as compounded medium — hydrated and demonstrated similar solubility and turbidity (not shown) to that of a traditional (noncompounded) base medium powder. Final (1×) liquid medium containing all components met a theoretical/target concentration for components tested (Table 1), including
amino acids determined by high-performance liquid chromatography (HPLC)
recombinant human (rHu) insulin determined by HPLC
trace elements and metals determined by inductively coupled plasma optical emission spectrometry (ICP-OES).
After hydration, pH levels were 7.50 and 7.56 for noncompounded and compounded media, respectively — with a target range of 7.20–7.60. Osmolalities were 346 and 334 mOsm/kg, respectively — with a desired range of 310–360 mOsm/kg (Table 1). These results demonstrate similar solubility and composition in liquid form from both preparation methods. They also show no apparent problems from blending and/or milling of the compounded dry-media components.
Performance: The compounded medium performed as expected in 1-L perfusion bioreactors. Cell culture growth and metabolism were comparable, as was production of recombinant product (titer) regardless of the media preparation method used (Figures 2 and 3). Comparable activity (confirmed through potency studies, not shown) indicated comparable PQAs for the recombinant protein made using both noncompounded and compounded media.
Preliminary media stability and performance (e.g., growth promotion) studies indicated no need for changes to storage temperature or other conditions for the compounded medium. Even labile components such as insulin appeared to be unaffected by inclusion in the compounded formulation. We saw no indications of undesirable interactions among the compounded dry-medium components (including those that previously had been added separately) at standard dry-media powder storage conditions (2–8 °C). Other important specifications were comparable too: e.g., bioburden, endotoxin, moisture, particle size, and solubility (not shown).
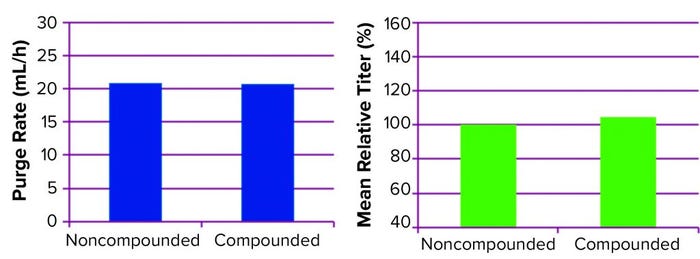
Figure 2: Media were prepared the standard way using base media powder and sequential supplements addition or using compounded media powder with only FeSO4–EDTA addition and used in 1-L perfusion bioreactor runs. Purge rate (an indicator of cell growth, left) and mean relative titer (right) remained unchanged.
Discussion
Using compounded dry-powder medium formulation significantly reduces complexity and cost while streamlining the media-batching process with safety and efficiency. Key benefits especially significant in a large-scale/commercial manufacturing environment include enhanced safety, reduction of waste and process complexity, and lowered capital requirements.
Safety Enhancements: Including insulin in the compounded media powder eliminates the need to prepare it in solution form before media batching. Because insulin solution requires hydrochloric acid (HCl) for preparation, the operation normally requires operators to work with a fume hood using additional protective measures (e.g., wearing respirators), thus presenting some risk to operators. That step is no longer necessary. Our new process also enhances ergonomics, eliminating several operational steps by consolidating individual component additions into the basal medium powder.
Reduced complexity saves costs and labor. Eliminating some addition steps streamlines operations (e.g., without time lost to waiting for individual components to mix/dissolve before proceeding to the next step). This also yields fewer batch-record entries and minimizes verification steps, benefits supply chain management with fewer enterprise resource planning (ERP) items, and improves warehouse management by decreasing the need for storage space. Requiring fewer items (individual media components) to be supplied, tested, and inspected (both by suppliers and in house) also provides significant cost reduction.
Working capital needs are lowered by transferring ownership of (currently site-stored) expensive components such as insulin to a medium supplier. That also curtails some procurement, second-supplier management, and batch-issuing activities, as well as tracking of individual medium components. The number of raw-material inspections and in-house testing operations that have to be performed on components added separately into media also decreases.
Waste Reduction: Packaging of components added separately does not always match batch-preparation size. So the original process had inherent waste that added to both cost and the overall environmental impact of our operations. We eliminated that waste by further compounding most such components.
Product Supply: By adding fewer components individually, we could minimize chances for operator mistakes. That not only saved raw-material costs, but also, and more important, helps to assure smooth production and reduces the number of investigations called for if deviations in good manufacturing practice (GMP) operations occur. An investigation can require holding product from commercial release because product made by a flawed process cannot be released until problems are resolved — such products might even need to be destroyed. Decreasing opportunities for errors thus is also important to ensuring continuous product supply to patients. Using compounded media is more amenable to continuous operations, of course, including a continuous media-batching process.
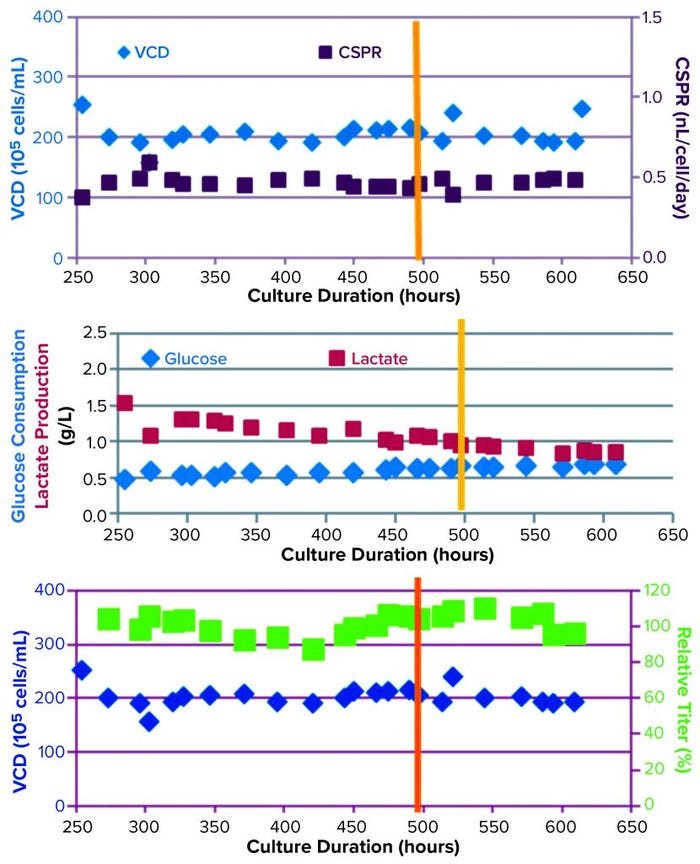
Figure 3: After about 500 hours (orange yellow bars), the 1-L culture was shifted from media prepared from noncompounded dry powder to media prepared from compounded dry powder. No change was apparent in cell culture performance and expressed protein titer after that shift. Viable cell density (VCD), cell specific perfusion rate (CSPR), trends of glucose consumption and lactate production, and relative titer all were apparently unchanged.
New Efficiencies for Continuous Biomanufacturing
Improvements in the efficiency of media preparation are an ongoing effort. Industry developments such as the use of bulk liquids instead of dry powders are gaining some momentum, especially in precommercial stages, where volumes are relatively low (7). However, liquid media are significantly more expensive than powdered media and would call for a paradigm shift in the supply chain (e.g., trucking ready-to-use media) at larger scales. That could present a logistical challenge to safeguarding continuous operations (such as commercial-scale perfusion) because of the ongoing large volumes needed to supply continuous cultures.
Other advancements — e.g., in dry-powder dissolution such as in Thermo Fisher Scientific’s Advanced Granulation Technology (AGT) (8) products — could offer an alternative approach to realizing many of the benefits discussed here with compounded media (6). An exciting aspect of such developments is that through using concentrates and in situ hydration, a truly continuous media preparation operation has become feasible even for large continuous-mode commercial scales. That brings the biopharmaceutical industry closer to implementing an end-to-end, closed continuous-manufacturing process: from media preparation through cell expansion and perfusion culture to isolation and purification of drug substance.
Acknowledgments
We thank Venkatesh Srinivasan (vice president of manufacturing sciences and technology at Bayer in California) and Helmut Brod (head of enzyme and fermentation technology at Bayer Technology Services in Germany) for their support of this project.
References
1 Scott C. Developments in Media for Culturing Cells. BioProcess Int. June 2005: S16–S27; https://bioprocessintl.com/category/2005/june-2005-supplement.
2 Shimoni Y, et al. Process Improvements Increase Production Capacity of a Legacy Product. BioProcess Int. 11(10) 2013: 26–31; https://bioprocessintl.com/upstream-processing/cell-culture-media/process-improvements-increase-production-capacity-of-a-legacy-product-347985.
3 Xiao Z, et al. Screening and Optimization of Chemically Defined Media and Feeds with Integrated and Statistical Approaches. Animal Cell Biotechnology: Methods and Protocols. Methods in Molecular Biology, Volume 1104. Pörtner R (Ed.), 2014: 117–135.
4 Shimoni Y, et al. Qualification of Scale-Down Bioreactors. BioProcess Int. 12(5) 2014: 38–45; https://bioprocessintl.com/analytical/upstream-development/qualification-of-scale-down-bioreactors.
5 Martinez B, Senter D. Innovative Strategies for Cell Culture Media Preparation. BioProcess Int. 17(11–12) 2019: 54–56; https://bioprocessintl.com/sponsored-content/innovative-strategies-for-cell-culture-media-preparation.
6 Shimoni Y, Moehrle V. Compounded Media Powder Formulation and Method of Preparation of Liquid Medium for Cell Culture. US Patent 10,227,559 B2, 12 March 2019; https://patentimages.storage.googleapis.com/b2/34/a8/1c7865a7c2f514/US10227559.pdf.
7 Langer ES, Rader RA. Powders and Bulk Liquids: Economics of Large-scale Culture Media and Buffer Preparation Are Changing. BioProcess Int. 12(3) 2014: 10–16; https://bioprocessintl.com/upstream-processing/biochemicals-raw-materials/powders-and-bulk-liquids-350510.
8 Advanced Granulation Technology (AGT). Thermo Fisher Scientific: Waltham, MA, 2020; https://www.thermofisher.com/us/en/home/life-science/bioproduction/gibco-bioprocessing/media-formats/agt.html.
Corresponding author Yuval Shimoni is a principal specialist in quality assurance at Bayer Healthcare LLC, 800 Dwight Way, Berkeley, CA; 1-510-705-5775; [email protected]. Volker Moehrle is a senior expert on cell and microbiology in pharmaceuticals product supply upstream technologies at Bayer AG, Leverkusen, Germany.
You May Also Like