Voices of Biotech
Podcast: MilliporeSigma says education vital to creating unbreakable chain for sustainability
MilliporeSigma discusses the importance of people, education, and the benefits of embracing discomfort to bolster sustainability efforts.
December 1, 2008
Cell culture automation has become more common in drug discovery and research applications, thereby freeing bench scientists from repetitive work as well as improving the consistency of their cell culture processes (1). Mammalian cell culture is used in the production of biopharmaceuticals, where developing a production cell line requires a large amount of repetitive manual work for bench scientists. With the increasing importance of biologics in today’s pharmaceutical market, throughput and efficiency are crucial in developing a production cell line. Identifying ways to automate parts of the process can increase throughput, efficiency, and consistency.
Cell culture automation for bioprocess development is an expensive investment, and many factors should be carefully considered before a company commits to a purchase. First, the work process should be clearly defined to justify the purchase. A company should also identify the time-consuming and repetitive steps of its process that would benefit from automation.
Once those potential steps have been identified, the company should carefully choose what type of automation to pursue. It could be an established system design, or it could involve building a custom, integrated design in consortium with an automation vendor. Automation also can be purchased in the form of smaller, stand-alone instruments.

WWW.PHOTOS.COM
In addition, a company exploring an automation purchase should carefully consider the potential antiquation of that system by new technology in the future. By defining the expectation for a system’s longevity, the company can make an educated decision about the type of system to purchase. A careful and strategic purchase of cell culture automation equipment can increase throughput in a development laboratory and bring a healthy return on the investment.
Identifying Processes for Automation
Before considering the purchase of automation for cell line development, a company should carefully decide where and when such automation would be appropriate in its process. A cell line development process should be well defined and standardized so that areas requiring high-throughput and repetitive work can be identified. Once those potential areas that could benefit from automation are identified, the cost of a system should be compared with the cost of laboratory staff. It is important to consider that the cost of automation entails its initial purchase as well as costs for set-up, training, operations, and maintenance. A company also must consider facility modifications that may be required to accommodate a new system along with the cost of consumables involved in using the system.
Several potential areas of a cell line development process can benefit from automation. They include screening cell lines in multiwell plates for productivity, limiting dilution or single-cell cloning, expansion/subculture of cell lines in multiwell plates, cell line growth and evaluation in T-flasks and shake flasks, process development in bioreactors, and cell banking.
The initial screening of cell lines is typically a high-throughput and repetitive process. Pfizer’s cell line development group in global biologics has a system from Thermo Fisher (www.thermofisher.com) that automates the screening of multiwell plates by enzyme-linked immunosorbent assay (ELISA). Flow cytometry can be used to automate single-cell sorting into multiwell plates. Other automation for screening and isolation of clonal cell lines include the Clonepix FL system from Genetix (www.genetix.com) and Cyntellect’s LEAP technology (www.cyntellect.com).
Pfizer’s cell line development group also has a system that was custom designed in partnership with Scinomix (www.scinomix.com) to automate shake-flask cell cultures. This system samples cultures, counts cells, and reseeds shake flasks. It also adds nutrient feeds to fed-batch shake-flask cultures (2). The system increases both consistency and throughput of passaging and feeding multiple shake flasks.
To address process development in bioreactors, Pfizer invested in a Simcell system from Bioprocessors Inc. (www.bioprocessors.com). It was designed to mimic the growth of cell lines in stirred-tank bioreactors by growing them at very small volumes (∼700 µL) on a chip called a microbioreactor array (MBA). Pfizer has paired this unit with an automated analytical system to enable the testing of potentially hundreds of different growth conditions to optimize cell growth and product expression in a microenvironment model system. This system is under evaluation as of this publication.
The Automation Partnership (TAP, www.automationpartnership.com) specializes in automation designed specifically for mammalian cell culture processes (3). TAP’s Cello system is designed for high-throughput mammalian cell culture in multiwell plates. It enables the selection of high-producing cell lines and automates their expansion and subculture in multiwell plates. The Sonata system is designed for automating shake flask cultures. It can culture up to 270 shake flasks simultaneously and actively manages cell counting, nutrient feeding, subculturing, sampling, centrifugation of samples, and sample storage. So it is similar to our custom shake-flask system from Scinomix except that the Sonata is a higher-throughput system with added capabilities. It also has a scheduling capability that allows it to initiate and run on weekends and holidays when technicians are not present. Consequently, the Sonata system is much more expensive than was our Scinomix workstation. And TAP’s Fill-It system automates the decapping, filling, and recapping of cryovials, providing a solution to generate more consistent cryovials for cell banking applications.
A number of areas in cell line development can be automated, and a number of automation solutions are commercially available or can be custom designed to meet a customer’s needs. Once the decision is made to pursue an automated solution, a company must carefully consider how to approach the purchase.
Approaches to Purchasing Cell Culture Automation
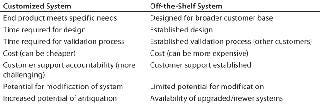
There are two general approaches to purchasing automation: as a customized system (customer designs a system based on needs, and an automation company builds it) or as an off-the-shelf unit designed by an automation company. Each approach has both advantages and disadvantages.
Some automation vendors will work with customers to design a system that specifically meets their needs. The advantage of a customized approach is that the resulting system is indeed specialized, and it can actually be a cheaper alternative to an off-the-shelf system. A custom design usually integrates a robotic arm with various other pieces of equipment. Since the system is customized, different instruments can be added or removed as a cell culture development process evolves.
The “customized” approach to designing automation presents challenges as well. If a company’s development process is changing and evolving rapidly, then a highly specialized system can quickly become obsolete. Although equipment can be upgraded and replaced on a customized system, keeping up with changes in a constantly evoloving process is time consuming, costly, and often difficult.
Decision-makers must realize that all automation takes time to implement, especially custom-designed systems. In fact, it can take up to a year from the time of installation to get a highly customized system fully operational. This includes installation, training, working out software/hardware issues, and validating system performance. And that one-year timetable does not even include the time it takes to initially design the system. Perhaps the most challenging aspect of making a successful investment in automation is accurately defining the expectation for longevity and assessing the risk of antiquation by new technology or modified work processes.
Another disadvantage of the “customized” approach is the challenge of clearly defining who will provide support for the automated system. Several years ago, Pfizer worked closely with another company to design a customized system consisting of a robotic arm on a table alongside several instruments for interaction with that arm. When completed, the system contained instruments from six different companies (not including the designer of the system). In addition to the software that controlled the robotic arm, the main company wrote several other software scripts to enable the arm to communicate with those third-party instruments. Pfizer also wrote some software that completed the system integration. After this system was installed, Pfizer observed some software issues, and it took several months for the main company and a third-party vendor to conclude who was responsible for addressing them. Because of that delay, it took much longer than expected to repair the problem. When purchasing a highly customized system, it is challenging yet crucial to assign clear accountability and prevent such delays when the inevitable problems arise (4).
Many automation companies offer complete systems, which can be advantageous because hardware and software are already designed. Most such systems have been tested and their issues have been properly addressed, especially if they have been purchased and used by other companies already. These off-the-shelf systems can be brought online more quickly than custom designs. Although these are not designed exclusively by their users, their design often results from a consensus of customers. For example, the TAP’s SelecT system was designed by a consortium including high-throughput screening, lead optimization, and core cell culture specialists from GlaxoSmithKline, Merck, Bristol-Myers Squibb, and Pfizer.
A complete off-the-shelf system has some potential disadvantages too. Just like a custom system, it can become obsolete in an environment where work processes constantly evolve, although to a lesser degree. The potential for antiquation of an off-the-shelf system is partially mitigated by upgrades provided by its vendor. With any system purchase, however, it is crucial to assess the flexibility of the system. And off-the-shelf systems often cost more than customized systems, so companies operating on tight budgets might consider the customized option instead.
A third approach to acquiring automation is to purchase components rather than design or buy an integrated system. An integrated system (particularly if it is customized) consists of smaller automated instruments that work together. They can also be bought as stand-alone units. When a larger system composed of multiple instruments is integrated, a lot of development time goes into software design and implementation. If different instruments are purchased separately, the time and work involved in establishing an integrated system is circumvented. The main disadvantage to such an approach is that it involves more work for bench scientists who must work with one instrument at a time rather than using an integrated system that interacts with several at once (Table 1).
Table 1: Factors to consider in comparing customized and off-the-shelf systems
Important Considerations
The largest risk to a good return on investment in automation is the potential antiquation of that equipment in processes that are constantly evolving. Therefore, when making a purchase, consider the following.
Clearly define the timeline for purchasing and installing automation. Remember that the timeline for a new automation system includes design, construction, validation, implementation, and training. It can be a long and detailed process, especially for a custom design.
Identify the quality of customer service and maintenance of an automation vendor. This is best accomplished by networking with other companies that have already bought systems from that vendor. Poor customer service and delayed repairs can make an automation purchase a very frustrating experience as well as a poor investment.
Determine the flexibility of a given system. If it has more flexibility in what it can do, then chances are better that it can be useful for a longer period.
Cell culture automation can be a good solution for high-throughput and labor-intensive areas of a development process. It can save a company money and bring more process consistency. However, an investment should be made only after careful thought and consideration. If a company clearly defines its process, identifies areas that could benefit from automation, and makes an educated choice on how to invest, automation can provide a healthy return on investment.
1.) Felder, R, and M. Kempner. 2002. A Review of Cell Culture Automation. J. Assoc. Lab. Automation:56-62.
2.) Zhang, L. 2008.. A Novel Integrated Robotic Workcell for Mammalian Shake Flask Culture Analysis.
3.) Aldridge, S. 2008. Solutions for Automating a Cell Culture Process. Gen. Eng. Biotechnol. News:26-27.
4.) Nie, D. 2008. Toys or Tools? Automation in the Drug Discovery Process. Am. Drug Disc.:32-36.
You May Also Like