- Bioprocess Insider
- Sponsored Content

Are you meeting quantitative safety standards for residual host cell DNA?Are you meeting quantitative safety standards for residual host cell DNA?
To realize the incredible potential of cell and gene therapies, scientists must overcome many challenges in creating these products, gaining regulatory approval, and ensuring their safe delivery to patients.
May 10, 2022

Sponsored by Bio-Rad Laboratories
To realize the incredible potential of cell and gene therapies, scientists must overcome many challenges in creating these products, gaining regulatory approval, and ensuring their safe delivery to patients. One persistent challenge is residual host cell DNA contamination, which can arise at multiple points throughout the development and manufacturing process.
HEK293 cells are a dominant platform for cell and gene therapy production because they are highly effective at producing large amounts of recombinant viral vectors. However, if traces of these host cells are not removed before the final treatment is administered to a patient, there can be dangerous immune responses or oncogenic effects. Regulatory consequences can also be devastating for biopharmaceutical companies that produce contaminated therapies.
To ensure patient safety when it comes to host cell DNA contamination, the Food and Drug Administration (FDA) published industry guidance for cell and gene therapy manufacturing:
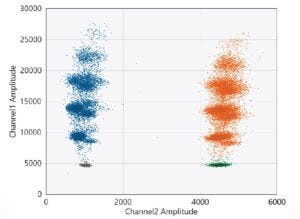
2-D amplitude plots showing data obtained by using the Vericheck ddPCR HEK293 Residual DNA Quantification Kit to test unextracted AAV sample with 25 ng residual HEK293 DNA contamination. The internal control is used as an inhibition control for unextracted samples. Plots show HEK293 signal in channel
1 (FAM) and internal control signal in channel 2 (HEX). The cluster in gray is double-negative for HEK293 and internal control, the blue clusters are positive for HEK293, the green cluster is single-positive for internal control, and the orange clusters are double-positive for HEK293 and internal control. The HEK293 Residual DNA Quantification assay is a 5-plex assay that detects five targets in the FAM channel. The higher amplitude FAM-positive clusters represent higher occupancy droplets that contain multiple HEK293 target molecules.
“We recommend that you limit the amount of residual DNA for continuous non-tumorigenic cells to less than 10ng/dose and the DNA size to below approximately 200 base pairs. If you are using cells that are tumor-derived (e.g., HeLa) or have tumorigenic phenotypes (e.g., HEK293, HEK293T) or other characteristics that may give rise to special concerns, the limitation of specific residual DNA quantities may be needed to assure product safety … Your tests should be appropriately controlled and of sufficient sensitivity and specificity to determine the level of these sequences in your product.”
To ensure adherence to these rigorous guidelines, biopharmaceutical manufacturers need cutting-edge solutions. The traditional method for quantifying residual host cell DNA is quantitative PCR (qPCR), which relies on a standard curve and presents other limitations such as nonspecific signals and lack of reproducibility. In addition, BioAnalyzer technology, which is widely used for DNA sizing, fails to deliver cell line-specific results and cannot analyze fragments larger than seven kilobases.
In contrast, Droplet Digital™ PCR technology (ddPCR™) delivers the high-quality data you need to meet FDA standards. ddPCR technology provides absolute quantification of target DNA without relying on standard curves. This capability makes the technique simpler, more precise, and highly sensitive.
The Vericheck ddPCR HEK293 Residual DNA Detection Kits are the first digital PCR HEK293-specific kits, providing two elegant solutions that enable precise quantification and determination of residual HEK293 DNA size on the same instrument with reproducible readouts. Validation studies indicated a limit of detection (LOD) of 0.1 pg/μl (3 wells) and a limit of quantification (LOQ) of 1 pg/μl (3 wells). These ddPCR kits also have low cross-reactivity and low false-positive rates, demonstrating over 95% specificity to HEK293 DNA when tested against Chinese hamster ovary, E. coli, and Vero cells
Droplet Digital PCR offers a simple, extraction-free workflow—minimizing hands-on time and sample manipulation. The kits accept a broad range of sample types, including in-process samples (cell lysates, cell culture media, sonicated samples, and adeno-associated virus [AAV] vectors) and the purified final product (phosphate buffered saline [PBS] with human serum albumin [HSA]). Finally, these tools also include positive control-based auto thresholding with regulatory compliant software. Scientists can perform automated data analyses using in-kit positive control with QX Manager version 1.2/QX ONE version 1.2 Software, which includes tools to help with U.S. FDA 21 CFR Part 11 compliance, offering audit trails with tracked protocol changes.
With these easy-to-use ddPCR assays, you can confirm that the precise amount of residual HEK293 residual DNA in your cell and gene products falls within the FDA guidelines for every batch. Empowered with the knowledge that your products are clear of dangerous contaminants, you can have greater confidence in your work.
Learn more about Bio-Rad’s Vericheck ddPCR HEK293 Residual DNA Quantification and Sizing Kit Assays.
About the Author
You May Also Like



