Technology Transfer for Outsourced Programs: Highlighting Upstream ConsiderationsTechnology Transfer for Outsourced Programs: Highlighting Upstream Considerations
January 29, 2025
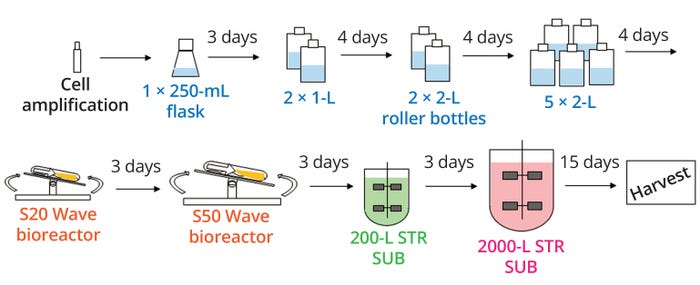
Figure 1: Example upstream cell-culture process to generate biopharmaceutical drug substance; STR SUB = Sartorius Biostat STR single-use bioreactor.
A proliferation of biotechnology start-ups and increased commercialization-capacity needs for biopharmaceutical companies have led to a paradigm shift from traditional in-house to external product-development and commercialization with the help of contract development manufacturing organizations (CDMOs). Such partnerships work on the underlying assumption that a process will be transferred successfully between companies and/or production sites, a process commonly referred to as technology transfer, which hinges on manufacturing requirements to meet clinical and commercial needs.
The Q10 guidance document from the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) provides a clear and comprehensive description of technology transfer as follows:
The goal of technology transfer activities is to transfer product and process knowledge between development and manufacturing, and within or between manufacturing sites to achieve product realization. This knowledge forms the basis for the manufacturing process, control strategy, process validation approach, and ongoing continual improvement. (1)
Technology-transfer processes require active management from small-scale process development through intermediary pilot scale and subsequent transfers to clinical and/or commercial manufacturing plants, either internally or externally.
Herein I focus on technology transfer of upstream production elements (from inoculation to harvest of production bioreactors) of the biopharmaceutical manufacturing. Figure 1 depicts an example upstream cell-culture process to generate a biological drug substance.
An Increasing Priority
Product Development Across Multiple Locations: The emergence of extremely large pharmaceutical companies with discrete “centers of excellence” in geographically dispersed locations has upended the classic model of one-stop shopping for all product-development functions. To ensure the smoothest path to market for a biopharmaceutical candidate, all involved must pay close attention to the principles of technology transfer.
Global Markets: Large companies are launching biopharmaceutical products around the world. Expanding into emerging markets requires technology transfer across locations that have distinct perspectives and approaches to knowledge transfer, product quality, regulatory compliance, other manufacturing requirements, and operations in their respective countries.
Mergers and Acquisitions: Realignment of global supply chains in response to mergers between key entities could drive transfer of products from one plant or site to another. In addition, companies often seek manufacturing efficiencies in their product portfolios. A structured and modular approach provides flexibility, consistency, and agility to serve evolving market needs and ensure technology-transfer success.
Figure 2 depicts a structured and modular technology-transfer approach. Process donors are primarily involved in modules A–C; process recipients are mainly involved in modules B–H (with bracketing). Foundational to those pivotal stages spanning a product life cycle is a modular yet flexible approach that is broken into discrete stages, with different needs for project evaluation and from different sets of stakeholders across both organizations. An iterative evaluation process requires combinatorial input from both management and program teams to derisk a project and help them determine an appropriate path forward.
Figure 2a: Benefits of a structured and modular technology-transfer approach based on market needs.
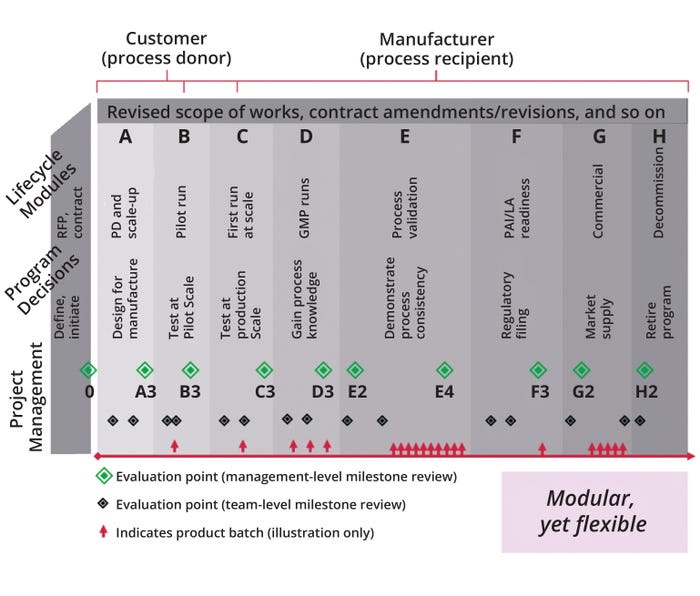
Figure 2b: A structured and modular technology-transfer approach based on market needs; GMP = good manufacturing practice, LA = launch, PAI = preapproval inspection, PD = process development, RFP = request for proposal.
Roadmap: The Transfer Plan
A biopharmaceutical company might engage a CDMO for technology transfer at different stages of a given product’s life cycle. Figure 3 (next page) identifies a few possible ingress points. They relate to an array of drug sponsors, from those requiring a CDMO for all process development and manufacturing activities to companies with their own established, commercial-scale processes needing transfer.
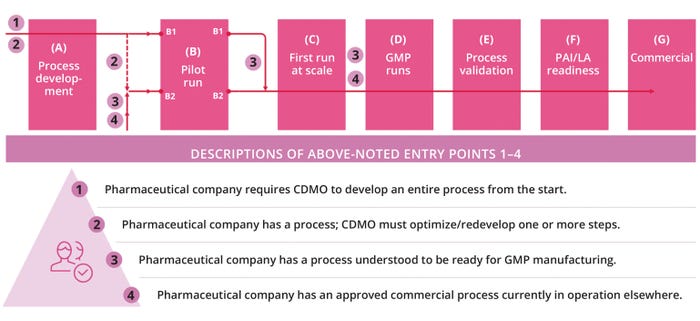
Figure 3: Entry points of technology transfer at different stages of the product life cycle; CDMO = contract development and manufacturing organization, GMP = good manufacturing practice, LA = launch, PAI = preapproval inspection.
Key to the success of a biopharmaceutical program is concurrent transfer of technology, process information, and skills gathered at every step of discovery, development, and commercialization. Technology transfer plays an important role in documenting all the active and tacit knowledge gathered at each step and provides an effective way to hand over the collected information to a receiving partner.
Figure 4 (next page) highlights five major activities carried out by a technology-transfer team for thorough transfer of a clinical or commercial project in facilities equipped with either single-use or stainless-steel (multiuse) equipment. Process design is underpinned by engineering, chemistry, and regulatory compliance to generate scalable and robust manufacturing processes with an eye to scope, budget, and cost of goods (CoG). Process materials are those raw materials typically derived from sources such as plants, animals, minerals, and chemical processes, any of which will be used from research and process development to manufacture of biopharmaceuticals. Materials used for final-product packaging also could be included in that category.
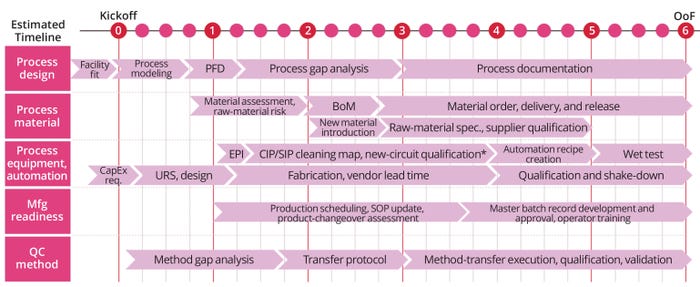
Figure 4: Major activities carried out by a technology-transfer project team for a thorough transfer; BoM = bill of materials, CapEx = capital expenditure, CIP = clean in place, EPI = engineering product identification, Mfg = manufacturing, OoF = out of freeze, PFD = process flow diagram, QC = quality control, Req. = requirements, SIP = steam in place, SOP = standard operating procedure, Spec. = specifications, URS = user requirements specification. * Methodical qualification and validation process needed when a new system is introduced to ensure that equipment and processes introduced are GMP compliant.
Process equipment and automation refers to the use of technology and automated systems to streamline, harmonize, and optimize different stages of a biomanufacturing process. Such systems help to mitigate the risk of errors and ensure compliance with quality standards.
Manufacturing readiness is a measure for assessing the maturity of manufacturing fit and supplier capabilities. This provides decision-makers throughout all levels with a common understanding of the relative maturity and attendant risks associated with manufacturing technologies, products, and processes under consideration. Such measurements often begin before process development and continue rigorously throughout each stage of a program’s life cycle. Manufacturing-readiness assessments can be used to define the current level of manufacturing maturity at any point, identifying potential risks and providing a basis for mitigating them.
In the figure, quality control method refers to a systematic process of testing and evaluating biotherapeutic products throughout production. Its goal is to ensure that products are safe for patient use while aligning to set quality standards.
Common Upstream Challenges and Solutions: Process development and manufacturing technical expertise drives effective technology transfers. To understand the important factors of a successful technology-transfer process, its key barriers need to be identified. The two main types of barriers are technical (complexity, consistency, and concreteness) and human (communication, motivation, and distance).
Scale-Up: One of the most critical aspects of upstream technology transfer is the need to operate fluidly across bioreactor scales, an endeavor that is made more difficult when scaling up to biomanufacturing. The ability to do so is underpinned by aligning tightly with established process parameters that are independent of scale (e.g., pH and dissolved oxygen, DO) while methodically evaluating product-quality attributes (PQAs) and characteristics. Without basic process understanding, expertise, and knowledge of key and critical process parameters (CPPs) and related technology-transfer elements, bioprocesses can be delayed significantly. In a worst-case scenario, a product might fail to launch. For upstream processing to generate drug substance, it is critical for developers to select, define, and (in some instances) optimize both media and cell-culture operations by leveraging quality by design (QbD) concepts and design of experiment (DoE) studies. It is also important to evaluate scale-dependent parameters and optimize for scalability and process robustness.
A common challenge encountered in bioreactor scale-up arises when vessel geometry changes from site to site or from small-scale process development to large-scale production. In addition, differences in mixing and aeration strategies between vessels can affect cell-culture kinetics. Efficient mass transfer is critical for cell metabolism, which is determined through volumetric mass transfer coefficient (kLa) experiments on bioreactors to determine equipment capability. Initial technology-transfer steps for a CDMO include aligning a customer’s specification for power per unit volume (P/V) and strategy for gas sparging.
Before running a customer process at scale, the customer-specific sparge strategy first is verified at small scale and then executed at a larger scale. Engineering runs often are performed at scale as well as before a program moves forward to drug-substance manufacture for either clinical or commercial supply. It is important to consider the scale differences in gassing efficiencies for controlling both pH and partial pressure of carbon dioxide (pCO2) levels, which can be sensitive to cell growth, process performance, and/or productivity. Development, process equipment, and automation knowledge — along with a robust at-scale strategy to control process attributes — drives success in scaling up for upstream technology transfer.
Another important aspect is a detailed and thorough gap analysis to help a receiving team understand the donor’s process-development history and differences between donor and operations, including instrument and equipment use. Examples to consider upstream include metabolite analyzers, cell-counting methods and instruments, differences in cell bags, single-use and stainless-steel bioreactor designs, and contact materials. Maintaining the same instrument and equipment types often is impossible in cross-company transfers. Gap analysis also should encompass comprehensive risk identification, assessment, and mitigation for all gaps. Those require action plans, including expected outcomes and due dates, to be established as part of technology transfer — which often may necessitate equipment wet testing and small-scale laboratory studies.
When customers focus on speed to market while desiring to improve a process for higher throughput (titer and yield) and lower CoG (e.g., by using less expensive media and resins), that places pressure on technology transfer that wouldn’t be there with a “locked process.” Accelerating timelines is possible for parallel process development and technology transfer, however, by leveraging a CDMO’s own platform-process knowledge, network capabilities, and expertise.
In one example based on Lonza’s CDMO experience (Figure 5), concurrent cell-line development, process development, and technology transfer were completed within a seven-month time frame to deliver a 75% improved expression titer performance. The client company was a medium-large enterprise with a monoclonal antibody (mAb) that was established using a Lonza platform process. The CDMO also generated and adapted a new cell line for a later platform process to meet the goal of improving titers for the customer.
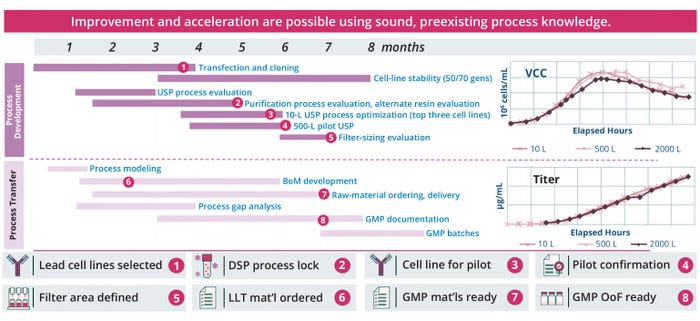
Figure 5: A Lonza case study for parallel cell-line development, process development and technology transfer; BoM = bill of materials, DSP = downstream processing, GMP = good manufacturing practice, LLT = long lead time, OoF = out of freeze, USP = upstream production, VCC = viable-cell concentration.
Key to that achievement was the parallel-processing approach taken. When feasible, such a strategy can save time and cost compared with sequential processing. Performing process-development activities such as transfection, cloning, and upstream process evaluations sequentially has been shown to increase the overall timeline by four to seven months. Similarly, waiting until the conclusion of purification-process and alternate-resin evaluations to initiate a 10-L upstream process optimization for the top three cell lines can add at least another four weeks. When a timeline of activities is assembled appropriately instead, that can assist in the management of both time and risk by making critical information available for clarifying, refining, and informing aspects of phase-appropriate goals. Each step makes use of knowledge from previous steps and iterative evaluations of risk along the way, and data generated from activities described above enhance the knowledge base of the overall technology-transfer package.
The last important factor to consider is a well-characterized and developed scale-down model for large-scale bioreactors. The case study in Figure 5 also demonstrates consistency from laboratory to pilot and good manufacturing practice (GMP) scale in both cell-growth and expression-titer attributes.
The scaled-down model of a production bioreactor should have a similar design and capabilities to the full-scale production vessels. For qualification studies, scale-independent variables typically are operated in scaled-down bioreactors at proposed target process values for commercial operations. Such parameters include, for example, pH, temperature, DO, culture duration, and the integral of viable-cell concentration (iVCC). The latter is a calculated metric of the effective working time of viable cells over a given period.
For scale-dependent parameters — e.g., agitation, gas-flow rates, pressure, volume, and pCO2 — operating conditions are established at small scale to match the full-scale process performance. For assessing the comparability of bioreactor performance and product quality across scales, a principal component analysis (PCA) model can be helpful. PCA transforms a large number of possibly related variables into a smaller number of uncorrelated variables (principal components) that are formed with different loadings of uncorrelated variables.
The strength of PCA is its ability to reveal the internal structure of data to explain variances. Such a multivariate approach provides a powerful means to assess whether the correlation structure between key performance and quality attributes in the scale-down model is comparable to that of its full-scale counterpart. The resulting model will be more sensitive than what can be achieved with univariate comparisons — e.g., t-tests — because it can detect observations that don’t fit predicted response patterns while offering fewer false-positive signals. Thus, the successful outcome of multivariate analysis lends credibility to
• the applicability of small-scale bioreactors as acceptable scale-down models for commercial production bioreactors
• a validation strategy that relies more on continuous process verification (CPV) than a minimum number of “validation batches,” which has been practiced historically.
Equal Involvement and Ownership of a Program: Success in technology transfer requires high motivation, good communication, and low equivocality of the project. A well-defined communication plan must be established as early as possible in the program, with defined and specific roles for every person involved. A “project champion” (with decision-making authority) is necessary to facilitate such regular communications. Effective engagement can be enabled further if both product developer and manufacturing partner begin working together as early as possible in a program. Early collaboration and involvement during project inception lead to a sense of ownership, thereby maximizing results. A client company having a “person in plant” at a CDMO is a key success factor for making and maintaining connections. Such an arrangement facilitates smooth and expeditious decision making.
A clear and effective governance structure is the primary enabler of technology-transfer success. Figure 6 provides an example of such a structure for technology transfer into CDMOs. Factors affecting success in technology transfer include business aspects such as choosing a contract manufacturer and establishing metrics for success at program initiation. But effective project planning and execution should be underscored. For instance, a governance team should discuss roles and responsibilities and assign both core and extended team members accordingly, defining the expected participation for each level. The team also should identify a keeper of the project plan, who can request technical reports and documentation for formal review of data by advanced team members in early technical and research discussion.
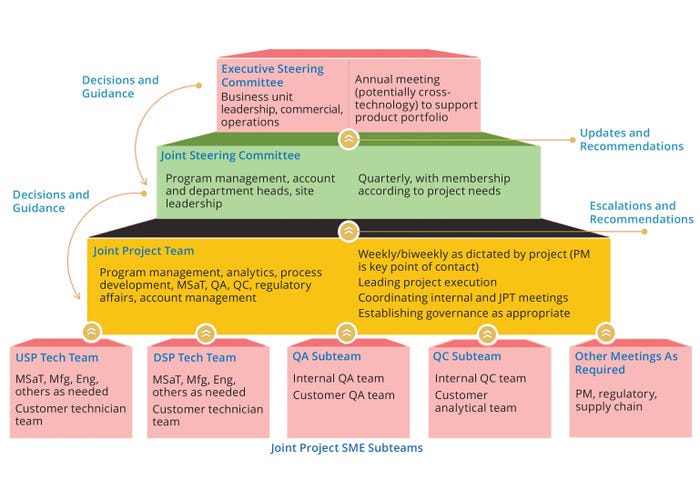
Figure 6: Example of governance structure for technology transfer into contract development and manufacturing organizations (CDMOs); DSP = downstream processing, Eng = engineering, JPT = joint product team, Mfg = manufacturing, MSaT = manufacturing science and technology, PM = project management, QA = quality assurance, QC = quality control, SME = subject-matter expert, USP = upstream production.
Subject-matter expert (SME) subteams are important to an effective governance structure and central to an overall joint-project team. Subteam membership is often cross-functional — e.g., with representatives from upstream, downstream, quality assurance (QA), and quality control (QC) groups — and such teams are responsible for leading activities that are integral the progression of a program. A joint steering committee should include program-management, department-head, site-leadership, and account-management personnel who meet quarterly. At the helm of each project is an executive steering committee made up of the business-unit leadership and commercial operations representatives. That group convenes annually to support a portfolio of products with the potential for cross-technology activities.
A Path to Mutual Success
Many biopharmaceutical product developers need a CDMO partner with the technical expertise to deliver scalable, robust, and reproducible processes to generate products that will be suitable for use in clinical studies and commercial sales. Foundational to a successful technology transfer from sponsor to partner are the right team, a sound plan, and a well-defined manufacturing process. Experienced teams at both companies will help to ensure that critical process fit and gap analyses are conducted and that suitable mitigations are proposed to address gaps in process operational fit. A roadmap also must be created based on clear understanding and oversight of project goals.
Project teams must be able to flex because challenges undoubtedly will arise over time, particularly as molecular formats and related processes become more complex. Leveraging good project-management skills such as proper documentation will be central to capturing important elements of a given transfer and will pave the path to creating and optimizing reproducible processes, no matter the complexity of the associated technologies. The ability to maintain an end-to-end view of technology transfer and use that understanding to balance competing priorities effectively enables teams to drive for the best overall value realization to advance biopharmaceutical therapies through development.

Mind the Gaps: Why a New Template Is Needed by Brian Gazaille (BPI managing editor)
At the September 2024 BPI Conference and Exhibition in Boston, MA, Nikki Nogal participated in a panel discussion about accelerating cell-line development (CLD) and a roundtable discussion about cell-culture processes. I caught up with Nogal after her sessions to learn about her experiences in technology transfer of upstream operations and about how her experiences shaped this article (accepted for publication in August 2024). She currently serves as global director of technical and chemistry, manufacturing, and controls (CMC) programs at a global contract development and manufacturing organization (CDMO); her technology transfer experience began at drug developer Gilead Sciences. Having worked with both process donors and adopters has given her valuable perspective. Below is an excerpt from our conversation at the BPI event.
What difficulties arise during upstream technology transfer? One challenge involves identifying sites that are sufficiently equipped to run a process. For instance, scientists at a sponsor site might use a hemacytometer to count cells at 1000-L scale. During transfer, a contract partner might need to run that process at 2000-L scale, and the adopting site, which could be located on the other side of the world, might use an automated cell-counting system. Such equipment differences can present challenges — e.g., for cell-count comparability across instruments. And many activities hinge on cell counts. They serve as triggers for feed protocols, temperature and pH shifts, and other process parameters. Cell counts will need to be comparable.
Many factors can create fitness gaps between donor and adopting sites. Consider equipment qualification: Instruments could be qualified for specific ranges at one site, whereas the receiving site might be unable to find that equipment, let alone ensure qualification for the same ranges. Some gaps are more critical than others are, but if I find gaps in critical process parameters (CPPs) across sites, then I begin to worry. An upstream example is vessel agitation rate. Vessel aspect ratios change by scale. In turn, mixing dynamics will change, and such differences can alter the cellular microenvironment, with cells reacting to culture conditions with shifting expression productivity and product quality.
What goes into gap analysis, and what factors complicate such evaluations? Gap analyses can differ among and even within organizations. The industry is beginning to find that sites are not using the same tools and criteria. That must be addressed. To that end, I have been involved in creating a gap-analysis template that is site agnostic. So when we conduct gap analysis at sites A and B, we can be assured that all operators, engineers, and scientists are examining the same parameters at both facilities. Having a common document such as a process-fit operational template can help us to ensure that we are capturing gaps at all evaluated sites.
How difficult is it to align stakeholders about processes and transfer protocols? There can be palpable challenges to getting everyone aligned, especially if the sites involved are geographically dispersed. That is true for sponsor–manufacturer relationships and even for a company’s internal operations. It comes down to company culture and practices. Some teams will be willing adopters when changes are needed to accord with best practice; other teams will dig in their heels and say that they have always done an activity in a certain way, sometimes despite a laundry list of associated process deviations. That attitude is not uncommon, unfortunately.
What do manufacturers need most from sponsors during transfer processes, and what can CDMOs do more effectively for their customers? Transparency is foundational to successful technology transfer. We need sponsors to tell us more about their processes than they generally do: Is a process susceptible to protein aggregation or clipping? What factors need to be monitored during cell culture? I always ask customers for CLD reports so that our manufacturing group can observe how clones behave during processing, comparing the trends and kinetics seen in our laboratories and in earlier studies. Then, we can identify concerning trends.
Meanwhile, manufacturers need to be flexible. Many CDMOs offer rigid platforms. We need to be comfortable with some level of bespoke processing. Especially as the biopharmaceutical industry moves from monoclonal antibodies (mAbs) to more complex molecular formats, we manufacturers need to be willing and able to pivot — e.g., to incorporate new or different bioanalytical testing to provide early screening. Sometimes, it might behoove us to do a little more science than is specified in a platform to understand particular process challenges, as opposed to barreling down the road with a process and realizing too late that a cell line exhibits a major protein-clipping issue or high aggregation propensity.
What other considerations do you have for technology transfer? I often wrestle with questions about timelines: How can we perform effective transfers more quickly than we have been doing? From the perspective of a transfer acceptor, the answer to that question lies in understanding internal capabilities. I also recommend that technology-transfer teams prioritize facts over feelings. If a process requires a lot of tinkering during scale-up, then something about it is insufficiently robust. Some people might try to provide justifications about how well-characterized a process is, but stakeholders need to take their blinders off so that they can move forward proactively.

Reference
1 ICH Q10. Pharmaceutical Quality System. US Fed. Reg. 74(66) 2009: 15990–15991; https://database.ich.org/sites/default/files/Q10%20Guideline.pdf.
Further Reading
Chattaway T. Unraveling the Complexities of Technology Transfer. BioProcess Int. 18(9) 2020: https://www.bioprocessintl.com/contract-services/unraveling-the-complexities-of-technology-transfer.
Chaudhry MA. Lessons in Bioreactor Scale-Up: Part 1 — Exploring Introductory Principles. BioProcess Int. 22(1–2) 2024: 22–27; https://www.bioprocessintl.com/bioreactors/lessons-in-bioreactor-scale-up-part-1-mdash-exploring-introductory-principles.
Chaudhry MA. Lessons in Bioreactor Scale-Up, Part 2: A Refresher on Fluid Flow and Mixing. BioProcess Int. 22(6) 2024: 32–40; https://www.bioprocessintl.com/bioreactors/lessons-in-bioreactor-scale-up-part-2-a-refresher-on-fluid-flow-and-mixing.
Chaudhry MA. Lessons in Bioreactor Scale-Up: Part 3 — Experimental Determination and Application of Oxygen Mass- Transfer Rate, Mass-Transfer Coefficient, and Oxygen-Uptake Rate. BioProcess Int. 22(9) 2024: 26–33; https://www.bioprocessintl.com/bioreactors/lessons-in-bioreactor-scale-up-part-3-experimental-determination-and-application-of-oxygen-mass--transfer-rate-mass-transfer-coefficient-and-oxygen-uptake-rate.
Chaudhry MA. Lessons in Bioreactor Scale-Up, Part 4: Physiochemical Factors Affecting Oxygen Transfer and the Volumetric Mass-Transfer Coefficient in Stirred Tanks. BioProcess Int. 22(10) 2024: 33–39; https://www.bioprocessintl.com/bioreactors/lessons-in-bioreactor-s-scale-up-part-4-physiochemical-factors-affecting-oxygen-transfer-and-the-volumetric-mass-transfer-coefficient-in-stirred-tanks.
Chaudhry MA. Lessons in Bioreactor Scale-Up, Part 5: Theoretical and Empirical Correlations for Predicting the Mass-Transfer Coefficient in Stirred-Tank Bioreactors. BioProcess Int. 23(1–2) 2025: in press.
Chaudhry MA. Lessons in Bioreactor Scale-Up, Part 6: Dissolved Carbon Dioxide and Its Impact on Cell-Culture Systems. BioProcess Int. 23(4) 2025: accepted.
Shimoni Y, et al. Qualification of Scale-Down Bioreactors: Validation of Process Changes in Commercial Production of Animal-Cell-Derived Products, Part 1 – Concept. BioProcess Int. 12(5) 2014: https://www.bioprocessintl.com/bioreactors/qualification-of-scale-down-bioreactors-validation-of-process-changes-in-commercial-production-of-animal-cell-derived-products-part-1-concept.
Shimoni Y, et al. Qualification of Scale-Down Bioreactors: Validation of Process Changes in Commercial Production of Animal-Cell-Derived Products, Part 2 – Application. BioProcess Int. 12(5) 2014: https://www.bioprocessintl.com/process-development/qualification-of-scale-down-bioreactors-validation-of-process-changes-in-commercial-production-of-animal-cell-derived-products-part-2-application.
Based in San Diego, CA, Nikki Nogal is global director of technical chemistry, manufacturing, and controls (CMC) for Lonza AG; [email protected]. A shorter version of this article was published online: Judd N, Liu M, and Nogal N. Considerations for Successful Tech Transfer of a Biologics Upstream Process. Euro. Pharm. Rev. 29(5) 2024: 2–6; https://dam.lonza.com/dmm3bwsv3/assetstream.aspx?assetid=25640&mediaformatid=10061&destinationid=10016&cat=Biologics.
You May Also Like






