- Bioprocess Insider
- Sponsored Content

Overcoming Lipid Nanoparticle Delivery Challenges with Low-Volume Subvisible Particle MeasurementsOvercoming Lipid Nanoparticle Delivery Challenges with Low-Volume Subvisible Particle Measurements
When it comes to successful drug treatment, the key lies not only in the active drug but also in the technology used to deliver it.
April 12, 2023

Sponsored by Halo Labs
When it comes to successful drug treatment, the key lies not only in the active drug but also in the technology used to deliver it. Ensuring drugs reach their target effectively is paramount, yet this is often easier said than done.
Fortunately, advances such as lipid nanoparticle technology (LNPs) provide a promising solution that can deliver large payloads, exhibit low immunogenicity, and enable manufacturing scalability. But there are still hurdles to overcome.
Like all biologics, LNPs can be physically unstable1, aggregate, form subvisible particles (SVPs), and thus need to be properly formulated and evaluated for physical and chemical stability.2 Traditional methods don’t provide the accuracy, speed or flexibility required. That’s where the need for ultra-low volume, SVP measurements come into play.
Characterize Subvisible Particles with Confidence
To help address this critical need, we’ve developed Aura® GT; a game-changing technology that provides an easier way to evaluate LNP quality and ensure the success of your drug delivery projects. Aura uses Backgrounded Membrane Imaging (BMI), a contemporary form of membrane microscopy that enables high throughput, low volume subvisible particle characterization.
And, by leveraging SYBR™ Gold assay via Fluorescence Membrane Microscopy (FMM), Aura GT allows you to assess both the stability and purity of samples in high throughput, low volume testing, enabling accurate LNP characterization at earlier stages, from initial development all the way through to final product release.
Aura GT’s SYBR Gold assay characterizes nucleic acid escape in stressed LNP samples and quantitatively analyze the stability and purity of these samples.
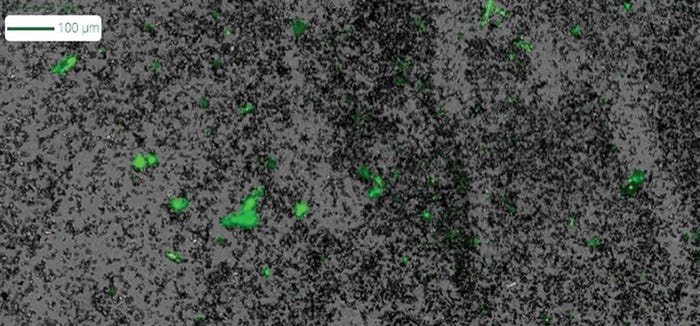
The challenges of accurately predicting quality and stability for lipid nanoparticles can be addressed with the help of Aura GT. By measuring LNP quality with as little as 5 μL of sample, Aura GT provides researchers and developers with unprecedented control over their drug delivery process – allowing them to monitor and evaluate subvisible particles with unparalleled accuracy.
References
Ball RL, Bajaj P, Whitehead KA. Achieving long-term stability of lipid nanoparticles: examining the effect of pH, temperature, and lyophilization. Int J Nanomedicine. 2016 Dec 30;12:305-315. doi: 10.2147/IJN.S123062. PMID: 28115848; PMCID: PMC5221800.
Trenkenschuh E, Savšek U, Friess W. Formulation, process, and storage strategies for lyophilizates of lipophilic nanoparticulate systems established based on the two models paliperidone palmitate and solid lipid nanoparticles. Int J Pharm. 2021 Sep 5;606:120929. doi: 10.1016/j.ijpharm.2021.120929. Epub 2021 Jul 23. PMID: 34303819.
About the Author
You May Also Like



