
Celltrion has inked a deal with iProgen Biotech to develop a pipeline of antibody-drug conjugates (ADCs).
While best known for its portfolio of marketed biosimilars, Korean drugmaker Celltrion has been investing in its internal R&D for novel biotherapeutics and has five antibodies in development for diseases ranging from rabies to influenza.
Now the firm is aiming to develop up to four antibody-drug conjugates (ADCs), including one targeting breast cancer, through a partnership agreement with Canadian firm iProgen Biotech.
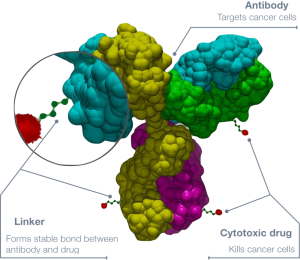
Image: Bioconjugator/creativecommons.org
Financial details have not been divulged, but spokesperson Gunn Lee told this publication the deal forms part of his Celltrion’s strategy in “not just developing and commercializing MAb biosimilars, but also researching new drug candidates,” which will drive future growth for the company.
Under terms of the deal, Celltrion will manufacture and supply four recombinant antibodies to iProgen, two of which will target HER2 and CD20, as well as provide support for the Chemistry, Manufacturing, and Control (CMC) activities for the IND application and the Phase I clinical trial. iProgen will conduct the Phase I trials.
ADC sector
Monoclonal antibodies linked to a cytotoxic agents, or ADCs, have shown great promise in targeting cancer.
However, there are only four ADCs currently available in the US: Roche’s Kadcyla (ado-trastuzumab emtansine), Takeda/Seattle Genetic’s Adcetris (brentuximab vedotin), Pfizer’s Besponsa (inotuzumab ozogamicin), and Pfizer’s Mylotarg (gemtuzumab ozogamicin).
(In 2010, Pfizer voluntarily withdrew Mylotarg after results from a post-approval clinical trial raised concerns about the product’s safety and efficacy, but the product was reintroduced in 2017.)
Despite the few marketed products, industry continues to invest in the sector with firms including Pfizer, Roche, Takeda and Daiichi Sankyo investing in R&D and manufacturing over the past few years. Outsourcing technology and manufacturing firms too have invested in the space, including WuXi Biologics/STA, Lonza, Piramal, ADC Bio and Althea.
About the Author
You May Also Like

schedl_b_and_w.jpg?width=100&auto=webp&quality=80&disable=upscale)
schedl_b_and_w.jpg?width=400&auto=webp&quality=80&disable=upscale)



