I am writing this shortly after BPI’s publisher Brian Caine and I conducted some interviews at an east-coast supplier company. Our experience there reinforced our rationale — from the start of this publication — for continually seeking ways to build on the collegial, collaborative nature of relationships in our industry. I’ve been covering this industry as an editor since 1989; Brian, Cheryl Scott, and Maribel Rios have been involved since the mid-1990s — so we have seen technologies come and go, trends justified, and ambitions disappointed. But because we ourselves don’t specialize in any one segment, we are allowed to see a broad view of the diverse technologies (and potential synergies among them) and continually celebrate the people who passionately support advancement of ground-breaking medicines.
That is what Brian and I were remarking on as we left the interviews. We have known some of you from the beginning of our time with this industry. In fact, it is unusual for one of us to walk into a company...
Niche-Disease: Pompe’s Disease
by Cheryl Scott
Pompe’s disease is a genetic disorder that causes a cellular buildup of the complex sugar glycogen. Its accumulation in organs and tissues impairs their ability to function normally. Since the disorder was identified in 1932 by Dutch pathologist J.C. Pompe, researchers have classified three forms of Pompe disease (also called glycogen storage disease type II or acid maltase deficiency): classic infantile-onset, nonclassic infantile-onset, and late-onset.
The disease is inherited in an autosomal recessive pattern. A defective gene is located on an autosome, and two copies of it (one from each parent) are required for the disorder to manifest. The parents of an individual with such a condition each carry one copy of the mutated gene but typically show no signs or symptoms of the condition.
Epidemiology:
Pompe’s disease affects about 1 in 40,000 people in the United States. It has been reported at different levels in almost all ethnic populations. As in all cas...
WWW.GRAPHICSTOCK.COM
Competitive pricing and continued cost pressures have contributed to the need for many US biopharmaceutical companies to outsource manufacture of active pharmaceutical ingredients (API) and finished products from countries with lower costs for labor, material, and equipment. The main benefit of doing so is lower costs of manufacturing with quality standards comparable to those found in the United States. India and China now account for 80% of API production. But those countries have received media attention because of biopharmaceutical manufacturing issues. In particular, some drug manufacturers in India and China have failed to ensure that quality control procedures are followed after receiving initial record approval from the US Food and Drug Administration (FDA) and do not pay close attention to ongoing quality control.
Quality is important to drug manufacturing. Poor-quality drugs are not readily apparent upon visual inspection. That being so, pharmacists will unwittingly fill pat...
https://bioprocessintl.com/wp-content/uploads/2015/04/032015_Jain.mp3
WWW.GRAPHICSTOCK.COM
Virtual companies are based on the model that all activities are outsourced. Such companies have no (or few) employees, occupy no laboratory space, and use contract service organizations for all activities. Over the past decade, several virtual biopharmaceutical companies have formed (
1
–
4
). They are primarily start-up ventures that use contract research organizations (CROs) for R&D and contract manufacturing organizations (CMOs) for product manufacturing. By contrast, a fully integrated biopharmaceutical company is based on the model that all activities are internal to the company. Such companies discover, develop, manufacture, and commercialize their products using internal resources.
Many biopharmaceutical companies follow a hybrid model: Most of their activities are internal, but they may in-license or purchase drug candidates from other companies and/or use a CMO for manufacturing. The hybrid model allows co...
https://bioprocessintl.com/wp-content/uploads/2015/04/032015-Vanderlaan_HamsterPLBL2.mp3
All recombinant protein biotherapeutics must be tested for the presence of residual host-cell protein (HCP) impurities (
1
–
3
). The most common analytical method for doing so is a polyclonal sandwich immunoassay. Polyclonal anti-HCP antibodies are selected to recognize the broadest population of HCPs possible. The immunogen and analytical standard are produced from a blank-run fermentation that mimics the production run but lacks the specific biotherapeutic protein. Because of the large number of impurities present in harvested cell-culture fluid (HCCF) that might copurify with the protein of interest, an HCP immunoassay must detect a broad spectrum of proteins. Thus, a limited number of anti-HCP antibodies in the immunoassay reagents will be directed against each particular protein impurity. Moreover, the assay results are immunologically weighted: Highly immunogenic HCPs will be detected more readily than non- or ...
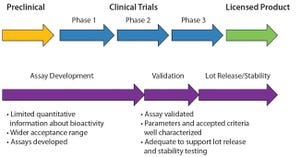
Compared with that for new drugs, biosimilar development faces significantly condensed timelines from cell line to first-in-human (FIH) trials. A biosimilar development program needs to accelerate quickly toward preclinical and phase 1 studies; phase 2 studies typically are not required because dose response and other patient-treatment concepts are already established by the original, comparator medicine. Phase 3 studies typically are limited to fewer patients, which ultimately shortens overall timelines and costs.
The key challenge remains: demonstrating comparability and high similarity based on in-depth analytics as outlined in a number of guidance documents from the European Medicines Agency (EMA), the US Food and Drug Administration (FDA), and the World Health Organization (WHO) (
1
–
3
). This often requires multiple iterations during early phase development compared to that for an originator product (
4
). An integrated approach to cell line development and early stage screening and process develop...
https://bioprocessintl.com/wp-content/uploads/2015/04/042015_MarcosSimon_BoB.mp3
WWW.PHOTOS.COM
The Bolt-on Bioreactor (BoB) project is an independent initiative to develop and commercialize a bioreactor for automated and efficient culture of adherent cells, especially in production of therapeutic cells and other biopharmaceuticals (
1
). After conducting thorough research on available culture systems for adherent cells, the BoB team believes that a successful alternative to existing devices must solve four major challenges. Addressed in the
first installment of this series
(
2
), the first challenge concerns volumetric productivity. The second challenge is automating culture processes, the third involves
containment and sterility
, and the fourth relates to process economics. This four-part series addresses each of those challenges in turn and describes design features that are incorporated into the BoB design for overcoming them.
The second challenge is to develop a system that can automate a continu...
Potency is a critical quality attribute of a biological product and is often determined by a biological assay (also called
bioassay
or
biopotency assay
). Specifically,
potency
is the biological activity or capacity of a product directly linked to its clinical efficacy. Potency tests are performed as part of product release, comparability studies, and stability testing. Nonbiological methods — which measure a product’s molecular or biochemical characteristics (e.g., ligand-binding assay) — have gained interest as replacements for often troublesome bioassays. Even with recent advancements in alternative methods, regulatory agencies expect manufacturers to make considerable efforts to first develop a bioassay, largely because of the method’s ability to directly assess a product’s biological function. The results of a bioassay can be used to gauge manufacturing consistency and product shelf life.
Whether based on cell culture, tissue models, or animals, bioassays are inherently variable because a living...
During the past decade, single‑use bioprocessing has emerged as a standard platform for current good manufacturing practice (CGMP) mammalian cell culture. Biomanufacturers have come to appreciate the benefits of lower capital and operating costs, reduced contamination risk, continuity from early development through manufacturing, flexibility, and sustainability (
1
).
Disposable cell‑culture vessels have gained wide acceptance because their performance duplicates that of stainless‑steel, fixed‑tank bioreactors, with which manufacturers have extensive experience. This is no accident: Single‑use bioreactors use stainless–steel engineering principles, particularly with respect to impeller rotation speed, agitation and aeration rate, reactor geometry, and so on. Retaining critical physical characteristics enables predictable, relatively problem‑free transitions from stainless to single‑use bioreactors.
Single‑use equipment is now widely used in mammalian bioprocesses outside of cell culture, including harvest...
As part of BPI’s “Ask the Expert” series, editorial advisor William G. Whitford (senior technical market manager for GE Healthcare Life Sciences) spoke with editor-in-chief Anne Montgomery and marketing and digital content strategist Leah Rosin on two separate occasions about issues related to culture media and expression titers.
Sourcing Serum-Free Media
Anne discussed cell culture media and process fluids with Bill in March 2014.
Whitford:
Things are advancing, and the industry is changing significantly. In general, we are using more structured approaches in management of cell-culture media and process fluids. Pharmaceutical sponsors are demanding that material suppliers provide a consistently available, compliant product that provides robust performance. More than ever, they are requiring that suppliers demonstrate more transparency in their establishment of effective and up-to-date supply-chain management techniques. They are expecting us to design and execute more formal validation strategies to ens...
The CMC Strategy Forums provide a venue for biopharmaceutical product discussion. They focus on relevant chemistry, manufacturing, and controls (CMC) issues throughout the life cycle of a therapeutic and thereby foster collaborative technical and regulatory interaction. Forum chairs share information with regulatory agencies to help them merge good scientific and regulatory practices. Outcomes of the forum meetings are published in
BioProcess International
and on the CASSS website. This process is meant to help ensure that biopharmaceutical products manufactured with advancing technologies in a regulated environment will continue to be safe and efficacious.
This special report series highlights five general subject areas that have been covered in the first 10 years of the CMC Strategy Forum series: quality by design (QbD) and risk management (
Part 1
and
Part 2
; manufacturing strategies; analysis and characterization; assays, biosimilars, and comparability; and process- and product-related impurities...
Both the United States and the European Union offer guidance on a life-cycle approach to process validation. This goes beyond the traditional three to five lots run at the center point of proposed ranges for operating parameters. New approaches leverage product design and process development information. They facilitate adapting the QbD paradigm to allow for a science- and risk-based selection of critical process parameters, key process indicators, and appropriate specification criteria. The number of runs for process performance qualification (PPQ) must be determined using a risk-based understanding and control of process variability.
This approach allows for more comprehensive use of multiple data sources to strengthen process understanding. Once process performance qualification has been executed, a stage of continued process verification begins for ensuring that a qualified control strategy is sufficient and that the process remains in a state of control. Following an appropriate time frame, process v...
The 15th WCBP CMC Strategy Forum, “Raw Material Control Strategies for Bioprocesses,” met on Sunday, 11 January 2009 in San Francisco, CA. This forum considered the design and implementation of control strategies for complex raw materials used in bioprocessing. Discussion focused on key approaches and application of risk assessment tools that can be used to identify and assist in mitigating potential safety and efficacy concerns that can affect the quality of biological products.
Two Sessions
To fully explore the topic, the forum first focused on recognizing and assessing potential risks associated with raw materials (RMs) and then applied those rationales to actual case studies with audience participation. The morning session began with an introduction to the topic followed by five presentations. Areas of discussion included how expectations for raw material control are evolving within changing regulatory and business paradigms including quality by design (QbD), counterfeiting, complex supply chains, and...
Multiproduct facilities are increasingly integral to corporate biologics network and supply chain strategies. Manufacturing capacity strategies ensuring appropriate facility design and procedural controls to manage the risks of producing multiple products are critical to the successful deployment of commercial and clinical supply plans.
A Chemistry, Manufacturing, and Controls (CMC) Strategy forum was held in Bethesda, MD, in August 2011 to highlight various challenges, risks, and control strategies associated with multiproduct facilities. Multiproduct strategies for the manufacture of a variety of product types at different life-cycle stages and potentially using different host cells were presented with case studies. Experts from both industry and global health authorities discussed facility design considerations as well as procedural controls such as cleaning validation and product testing. The importance of quality risk management (QRM) to multiproduct operations and controls was also discussed using p...
The importance of investing science and technology into drug product development has become evident as different product types, higher protein concentrations, and doses and requirements for improved delivery of biological drug products have increased. The need to give patients larger and more concentrated doses has challenged formulation scientists and driven development of new technologies that can deliver those doses. Delivery devices fall under device regulations and have distinctly different design, development, and validation requirements from those of protein drug products alone. The regulatory environment also has evolved, whereby a biological drug product (in even simple delivery systems) is now considered a combination product, making additional development consideration and additional requirements applicable.
A CMC Strategy Forum on drug products for biological medicines, including novel delivery devices, challenging formulations and combination products was held in July 2012 in Bethesda, MD. Th...