Biopharmaceutical companies face a broad range of challenges when developing new large-molecule products, including
Flexible Biomanufacturing
Patheon flexible biomanufacturing solutions offer a unique combination of expertise in development and manufacturing with advanced technological capabilities, innovative approaches, and flexible business models that can overcome challenges and reduce your risk.
Perfusion Processing:
Our expertise in mid-scale perfusion techniques allows for higher yields from low-titer cell lines as well as manufacturing of recombinant proteins with stability challenges, which result in higher product volumes at any given reactor size.
Single Use or Flexible Stainless Steel:
Single-use technology allows for faster, more efficient technology transfers; eliminates cross-contamination concerns; and simplifies management of your supplier relationship by streamlining your process. For processes that require stainless steel systems, our experienced staff achieves a 95% success rate to...
The pathway from conception to regulatory approval and commercialization of a cell therapy is long, complex, and resource intensive. To help inform numerous decisions along the way, an effective commercial manufacturing strategy for a cell therapy should be built on the four principles of what PCT calls development by design (DbD): quality, cost of goods (COGs), scalability, and sustainability. Proactively implementing a DbD strategy does not force a cell therapy developer to make a large manufacturing investment early in a project, but it does mean that the company must plan ahead. As a cell therapy moves through clinical development, working through the four facets of DbD at an early stage can provide significant cost and time advantages as well as effective management of comparability risk.
With DbD in mind, a prudent cell therapy developer will initiate and produce a strategic commercial manufacturing plan (SCMP) with the support of an experienced manufacturing partner. An SCMP provides a comprehensiv...
Rentschler is a full-service contract development and manufacturing organization (CDMO) and one of the leaders in the industry. Focused on the development and manufacturing of biopharmaceuticals in mammalian cell culture, we support our global clients through to market approval of their products. Delivering successful projects allows us to make an essential contribution to the global availability of biopharmaceuticals. Thanks to many years of experience, the high quality of our consulting services, and our flexibility, we can react quickly and effectively to clients’ requirements. Our success as a CDMO is underpinned by our focus on product quality, on-time delivery, and comprehensive and tailored project management. Founded in 1927, Rentschler is a privately owned medium-sized company headquartered in Laupheim, Germany.
InnovatIve Technology
Rentschler’s proprietary TurboCell™ mammalian expression platform enables the simultaneous production of up to 20 early stage drug candidate variants. The vector c...
Richter-Helm is a Hamburg, Germany–based contract development and manufacturing company with a proven 25-year track record, specialized in products derived from bacteria and yeasts. Count on us to flexibly provide a comprehensive range of services and customized solutions. Clients worldwide already have benefited from our commitment to good manufacturing practice (GMP) and total transparency. Our work focuses on recombinant proteins, plasmid DNA, antibody fragments, and vaccines.
Our seasoned, 160-strong team supports you with process development, supply of products for clinical trials, commercial production, in-house quality control (QC) testing, and QP release. We operate two GMP-compliant production plants with bioreactor capacities of up to 1,500 L.
Richter-Helm consistently works to the highest standards of pharmaceutical quality, as verified by major regulatory bodies (European Medicines Agency (EMA), the US Food and Drug Administration (US FDA), Agência Nacional de Vigilância Sanitária (ANVISA), an...
Vetter is an international specialist in the production of aseptically prefilled syringe systems, cartridges, and vials. We are a family-owned, independent company and do not manufacture our own drugs.
Resources for Every Stage of Growth
Vetter’s full portfolio of services includes dedicated resources for both clinical development and commercial manufacturing. In addition, we provide expert packaging technologies and solutions tailored to your product’s specific market needs.
Vetter Development Service
Planning for Success:
Preclinical through phase 3 is a pivotal and unpredictable period for new molecules. Vetter Development Service helps smooth the path to clinical trials with dedicated support for key stages of development, testing, clinical manufacturing, and regulatory approval. We also help you integrate thoughtful, efficient life-cycle solutions for long-term growth and success. Vetter development service provides
Vetter Commercial Manufacturing
Filling Your Potential:
Both precise manufacturing ...
Wacker Biotech is “The Microbial CMO” — the partner of choice for contract manufacturing of therapeutic proteins using microbial hosts. Our service portfolio covers molecular biology, process and analytical development, and the GMP production of biologics for clinical trials and commercial supply. Founded in 1999 as a spin-off from the Hans-Knöll Institute in Jena, we are a 100% subsidiary of Wacker Chemie AG since 2005.
The sites in Jena and Halle provide a complete range of services for the development and GMP-compliant manufacture of biopharmaceuticals. Two manufacturing lines with 300-L and 1,500-L fermentation vessels and matching DSP scales are available to suit all possible customers’ needs.
WACKER holds biomanufacturing certificates from the relevant authorities for both sites and follows the ICH Q7A guidelines for GMP-compliant production of biologics. Since 2012, the facility in Halle has been EMA-approved/FDA-inspected for the commercial production of Reteplase (trade names Rapilysin® and Retav...
Development of a single drug, whether it is a new chemical entity, biotherapeutic, or cellular therapy, requires significant investment of resources. Care must be taken to choose analytical methods that are fit for the purpose of monitoring products and contaminants in the process. With expertise in labeling and detection, Enzo offers solutions to help rapidly analyze protein stability and integrity for all your bioprocessing needs.
Figure 1: Broadest detection range from visible to subvisible particles
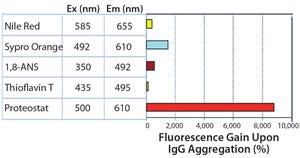
Protein Aggregation
The PROTEOSTAT® Protein Aggregation Assay (ENZ-51023) is a sensitive, homogeneous, fluorescence-based detection assay. The novel molecular rotor dye detects a broad range of different protein aggregates from visible to subvisible particles (Figure 1).
Figure 2A: Detects low % aggregate in concentrated samples
With convenient options for analysis, this high-throughput assay can be used on either a fluorescent microplate reader or flow cytometer. It provides a convenient, complementary ...
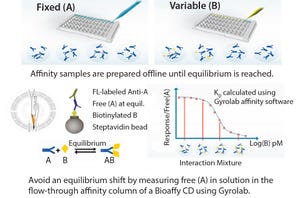
In the development of biotherapeutics, there is a need to quantitate antibody titer and to characterize antibody affinity against the target of interest. Such analyses are often challenged by the need for sensitivity, reproducibility, and higher throughput. Gyrolab systems automate and miniaturize affinity measurements and IgG titer quantification in a single platform using only nanoliter volumes with rapid time to results.
Figure 1: Gyrolab huIgG titer kits provide reliable measurement of human IgG titer over a broad dynamic range and with excellent reproducibility.
IgG Titer Immunoassays with Broader Ranges for Cell Line Development
The immunoassays used to determine IgG titer must have the flexibility to support a broad range of concentrations encountered from expression to production. Gyros recently launched ready-to-use kits to rapidly measure human IgG titer at a higher throughput using only nanoliter volumes for use on Gyrolab systems. The convenience and robustness of kits combined with the auto...
Host cell protein (HCP) levels in biologics are on the critical path for assessing product quality, and they pose a serious risk to drug safety. The challenge is to accurately quantify a complex mixture of HCP impurities, which vary in properties and abundance depending on cell line, media, and process parameters. HCP immunoassay analysis is based on polyclonal antibodies raised against HCPs from nontransfected cell lines.
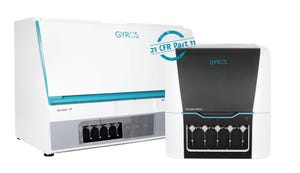
Automated immunoassays with high-throughput (Gyrolab xP workstation, left) or single-CD configuration (Gyrolab xPlore, right)
How well a particular HCP assay recognizes all proteins depends on how well the antibodies match the HCP composition, their abundance, and their affinities. To cover the diversity of HCP mixtures from different bioprocesses, Gyros has developed a panel of five Gyrolab CHO-HCP kits to increase the possibility of finding the assay that best matches a specific CHO-K1 process. A scouting kit simplifies selection of the optimal generic kit with broader dynamic ranges,...
Assessment of thermal stability parameters of biologics is an integral part of biopharmaceutical research. The ever-growing number of biologics in development pipelines worldwide demands rapid and precise methods to quickly screen large sets of conditions in an easy and straight-forward manner.
In a recent study, we compared two methods for detection of thermal unfolding transition temperatures (
T
m
) of a therapeutic monoclonal antibody (MAb): nanoDSF, which analyzes changes in the fluorescence emission properties of proteins, and differential scanning calorimetry (µDSC), which detects changes in the heat capacity of a protein solution upon unfolding. Both nanoDSF and µDSC provide precise and consistent data. But nanoDSF also overcomes several limitations of µDSC (e.g., low throughput and high sample consumption) and thus represents the ideal technology for rapid and precise thermal stability screening in biopharmaceutical development.
Prometheus NT.Plex nanoDSF instrument
In a second study we demonstra...

 +1
+1