Celonic’s CHOvolution™ kit comes with a wide range of exceptional services and solutions, on top of high-performance cells. In addition to robust and highly efficient technology, we offer excellent technical support, including comprehensive protocols, audits, tailored workshops, and a 24-7 information access Internet-based platform with a hotline.
For any drug developer or service provider, cell line development is one of the most challenging phases, especially when subsequent GMP compliance is required. To boost and support successful commercial introduction of biological drugs to the market, Celonic has developed CHOvolution™, a cell line kit that can be used for a broad range of applications ranging from non-GMP R&D testing to GMP development and commercial market supply, with a support system to help throughout every step of development.
Celonic: Beyond Manufacturing
Celonic AG is privately owned CDMO based in Basel, Switzerland providing comprehensive GMP development and manufacturing services for n...
In recent years, single-use bioreactors have gained more importance in animal and human cell culture. With its new line of BioBLU frigid-wall, stirred-tank, single-use vessels, Eppendorf offers premium solutions for microbial applications. In the following case study, reproducible process control was achieved with parallel-operated BioBLU 0.3f single-use and reusable glass vessels, both used in an Eppendorf DASbox® mini bioreactor system. Fermentation of
Escherichia coli
K12 led to very comparable results, thus proving tested single-use vessels to be an appropriate tool to accelerate microbial process development and shorten time-to-market in all industries related to microbial production processes.
BioBLU of 0.3f single-use vessel (left) and DASbox mini bioreactor (right)
IntroductIon
Single-use bioreactors are a suitable tool for time- and cost-effective bioprocessing. Minimized setup times, eliminated cleaning procedures, and reduced labor time can sustainably accelerate bioprocess development. In ...
Single-use technology has transformed the biomanufacturing industry, because single-use products provide significant savings over stainless steel infrastructure. Single-use technologies also create flexibility for biomanufacturers, enabling them to move from single-product processes to multiproduct facilities. As revolutionary and transformative as single-use technologies have been, the industry is on the cusp of another major shift, which comes with its own impressive benefits, but with some challenges as well.
Continuous and Batch Processing
Continuous processing has been used successfully for many years in a number of manufacturing industries such as in the production of food, chemicals, and steel. When applied to biomanufacturing and combined with single-use technology, continuous processing can offer much higher throughput and increased flexibility.
Batch processing has been the logical method in biomanufacturing for regulatory compliance and quality control. The batch method establishes clear bounda...
Fast and accurate determination of vaccine titer during manufacturing is important for understanding the performance of a development process and for scaling each process step. Although single radial immunodiffusion (SRID) has been the most commonly used technique for vaccine titer determination, it can be time-consuming and imprecise, requiring up to three days for results. The Pall ForteBio Octet® system offers a simple and direct method for measuring vaccine–antigen–antibody binding, capable of delivering high-precision analysis in as little as three hours.
Table 1: Comparison of Octet system with single radial immunodiffusion (SRID); B/Massachusetts influenza virus
Methods
Using the influenza virus as a model system, the relative standard deviation and dynamic range of a vaccine titer assay were measured with the Octet system and compared with results from using SRID. Polyclonal serum antibodies were loaded onto Octet biosensors, and their binding measured to epitopes presented by an inactivated virus...
Efficient expression of proteins is an integral part of the biopharmaceutical drug development process. With a range of expression systems, it is important to balance long-term manufacturing goals (e.g., cost of goods and productivity) while matching the expression system to suit the characteristics of a protein (e.g., product quality attributes and long-term stability). Finding the right expression system early on can make or break a therapeutic candidate as it moves from laboratory bench to clinical administration.
FUJIFILM Diosynth Biotechnologies has developed its pAVEway™ expression technology to support microbial expression of proteins. This platform technology incorporates three elements in the delivery of an
Escherichia coli
expression process: optimized expression vectors and control elements, preselected host strains, and platform development using a high-throughput approach for fast strain selection and/or design of experiment (DoE) approaches.
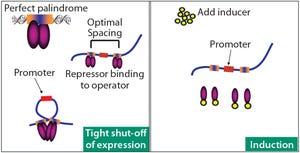
DNA looping in pAVEway™ expression technology is...
Today algae are commercially cultivated for pharmaceuticals, nutraceuticals, cosmetics, and aquaculture purposes.
The abundance of vitamins, minerals, and trace elements as well as the fatty acid profile and thickening and stabilizing functions of their polysaccharides make algae valuable. Algae also can be used to produce biodiesel, bioethanol, and biomass that can be burned to generate heat and electricity. Like plants, algae use sunlight during photosynthesis. Photosynthesis is an important biochemical process in which plants, algae, and some bacteria convert the energy of sunlight to chemical energy. Algae capture light energy through photosynthesis and convert inorganic substances such as CO
2
into simple sugars or oils using the captured energy.
KIT laboratory
Several factors (e.g., light, temperature, pH, nutrients, aeration, and mixing rate) can be measured to determine the growth rate of algae. To optimize yield, algae is cultivated in closed photobioreactors. Within those, process conditions c...
Many biopharmaceutical manufacturers are implementing single-use containment for transfer of media and buffer materials to prevent feedstock contamination and promote worker safety. But the type of containment system used can have a significant impact on productivity and profitability. Equipment should be evaluated and chosen carefully.
Pertinent Questions
Systems should be analyzed not only for their basic ability to contain powders of interest, but also for how efficiently they integrate into production process. For instance, could the system introduce productivity bottlenecks, increase material waste and the cost of raw materials, or complicate changeover of a line to new products? Is it robust enough to provide reliable transfer throughout the production run? And has it been designed specifically to contain and release powders? The answers to those questions are crucial to identifying and implementing a sound powder-transfer system.
Issues of Powder Handling
Media and buffer ingredients used in powd...
The need for a rapid turn-around in quantity (and the loss of technicians in many laboratories) has created a trend toward implementation of single-use vessels. Their advantages include no time lost in cleaning or sterilization (especially for bench-scale, autoclavable systems), no chance of inhibition or cross-contamination due to poor cleaning, and no need for cleaning and sterilization facilities in a crowded laboratory.
For production-scale steel bioreactors, fully automated clean-in-place (CIP) and sterilization-in-place (SIP) have been possible for decades. By contrast, bench-scale systems required considerable downtime for disassembly, cleaning, reassembly, autoclaving, and cooling before use — with no guarantee that each step was carried out correctly and reproducibly. An innovation from INFORS HT brings the advantages of CIP/SIP to bench-scale bioreactors, offering a true alternative to single-use systems for microbial applications.
Table 1: Sterility testing against a panel of highly resistant t...
For over 75 years, Kerry has earned its reputation for reliability and excellence in serving the biotechnology, pharmaceutical, and nutrition markets. We deliver innovative solutions to assist customers increase cell proliferation, extend cell viability, and increase target protein production in biotechnological production systems.
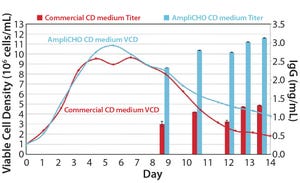
Figure 1: Viable cell density and IgG titer in CHO DG44 cell line
Every day, we expand our capabilities to meet the changing needs of the biotechnology market. Kerry’s products have evolved with market trends in cell culture over the past 50 years to fulfill our customers’ requirements and have grown to encompass modern plant-based hydrolysates, yeast extracts, recombinant proteins, cell-line–tailored supplement systems, and chemically defined media. Kerry has the vast global resources and technical platform to deliver consistent, high-quality products backed by unparalleled service, technical support, and formulation customization capabilities.
Chinese hamster ovary (CHO) cell...
During the fall of 2014, we received requests from a couple of large, single-use technology (SUT) system integrators asking whether we could develop a new tube clamp for their SUT assemblies and skids. There were several motivating factors behind these requests. As bioreactor skids became larger and larger (with thousand-liter and larger SUT containers), technicians had increasing difficulties assembling current clamps onto tubing. In some cases, technicians were lying on their stomachs and reaching under the bioreactors trying to install clamps. In other cases, they were standing on tall ladders or platforms reaching across the tank and container to access tubing.
PharmaLok™ tube clamps during the long-duration pressure test
After discussing the technicians’ needs in more detail, we came away with a list of challenging requirements for a potential new product. The number-one desire was for a clamp that can be easily opened and closed using just one hand. This would simplify assembly while technicians rea...
A finalist for the 2012 BPI Technology of the Decade Award, XCell™ ATF systems consistently yield high cell retention, concentration, and viability, enabling efficient and productive perfusion and
N
– 1 perfusion as well as cell banking and seed-train cell culture operations.
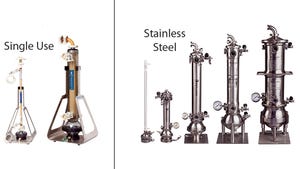
Figure 1: Scalable from laboratory to production: XCell™ ATF systems include the device and controller. XCell™ ATF single-use device (left) are available in two sizes. XCell™ ATF stainless steel device (right) are available in five sizes.
XCell™ ATF devices are now available in single-use format, in addition to stainless steel. The new single-use devices deliver 100% cell retention and laboratory-to-production scalability like their stainless steel counterparts but without the need for autoclave sterilization and lengthy setup times.
Figure 2: Alternating tangential flow (ATF) technology: XCell™ systems use ATF technology created by the action of a diaphragm moving upward and downward within a pump head, connected to a filter housi...
Modern, automated peptone production
Peptones from the Solabia Group represent the result of nearly 50 years of strategic activity and savoir-faire. As key components in industrial fermentation, they contribute to a range of products, from probiotics and vaccines to specific bacterial metabolites in cosmetics. Although they are often perceived as replaceable commodities with similar sounding names, that misconception can lead to significant problems. Peptones differ in sourcing as a function of a producer’s manufacturing experience, raw materials, and (most important) production site itself and its level of technical advancement. The more modern the site, the greater the likelihood that it can produce consistent, high-quality peptones.
Dual Production Sites and Animal Separation Set Solabia Apart
Solabia separates animal tissue processing from its plant and casein manufacturing, which is unique to the peptone industry and an important part of eliminating cross-contamination. Solabia’s production site for ...
Standardizing equipment in biopharmaceutical production streamlines operations, saving end users time and money. An area ripe for standardization is the use of genderless connections, those in which the connector halves are identical in design. Historically, single-use connections have involved male and female halves, but using two different parts can create several inefficiencies and complications, such as
By standardizing single-use genderless connectors, end users can accomplish more with fewer finished goods, save time, reduce overhead costs, and get their biopharmaceutical products to market more efficiently. Benefits include
Overall, genderless connector standardization will help bioprocessing facilities simplify their operations and implement single-use technology to its full potential.
Todd Andrews
is the global sales and business development manager, Bioprocessing at CPC, 1001 Westgate Drive, St. Paul, MN 55114; 1-651645-0091;
[email protected]
.
Clarification of fermentation broths is one of the most important steps in bioprocessing. The first purification step after fermentation is the cell harvest, which is designed to remove cells and cell debris as well as to reach maximum product yield in compliance with existing regulatory environments. Standard technologies (centrifugation, separators, membrane, and depth filtration) can no longer handle the high particle loads (>10
8
cells/mL) in an economical way.
Deciding on the right purification system involves addressing questions about process performance, economics, and existing regulatory requirements. Process performance challenges include higher and higher cell titers, cell debris content, scalability, and flexibility for process changes and future processes, higher product yields, and constant high-quality product flow for further downstream purification.
Alluvial filtration (see box below) is a well-established and economical method in pharmaceutical industries (plasma fractionation). Use of ...
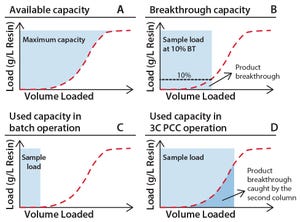
Interest in continuous bioprocessing is growing rapidly, and such an approach has shown to have a potential in providing gains in both productivity and savings in cost of goods sold. The ÄKTA™ pcc 75 chromatography system can be used for purification of target proteins in continuous downstream processes using periodic counter-current chromatography (PCC). PCC enables greater use of chromatography resin capacity, allowing sample loading to much higher levels than what is possible in traditional batch chromatography by using safety factors for loading (Figure 1). This study shows how the dynamic control functionality of ÄKTA pcc 75 can be used to adjust for variations in binding capacity of chromatography resin or for changes in feed composition when, for example, perfusion cell culture is being used. In this study, dynamic control was used for a protein A monoclonal antibody (MAb) capture step operated in a three-column PCC (3C PCC) setup.
Figure 1: Capacity use for standard and PCC; (A) total available ca...
In batch-mode chromatography, operating conditions do not allow full use of the total media capacity (
1
,
2
). Bio-Sequential Chromatography (BioSC®) is an open-loop process for the separation of multicomponent solutions (
3
). The unique column of the batch mode is replaced by a series of smaller columns (from two to six). This design maximizes the use of the media without product loss along the process sequences. The BioSC® Lab automated system is based on the multicolumn chromatography process, designed and provided by Novasep. Studies have been performed to demonstrate the benefits on IgG purity of a downstream process (DSP) monoclonal antibody (MAb) platform with two continuous chromatography steps (
4
). This note features the outlines of the method used and the qualitative and quantitative results obtained for the product critical quality attributes (CQAs) (Table 2).
Analytical Methods
Table 1: Batch and recipe targets generated by BioSC® Predict software
Feed Description:
IgG1 subtype MAb was p...
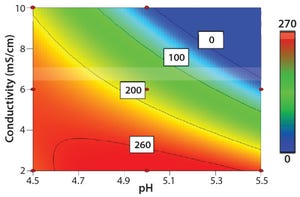
Cellulose is well known as a natural raw material that has mechanical strength, lower nonspecific adsorption, and good biocompatibility. In addition, cellulose particles have unique pore-size characteristics appropriate for chromatography of biopharmaceuticals. Ion-exchange chromatography (IEX) is an important step for biopharmaceutical manufacturing. Specifically, cation-exchange chromatography (CEX) can be used as a capture step in monoclonal antibody (MAb) purification. Recently, advanced IEX resins have been developed using polymer modification techniques. Initial screening of conditions such as pH and ionic strength is important for IEX resin. The objective of this study is to reveal the differences of adsorption properties in polymer-modified, cellulose-based CEX resins.
Figure 1A: Contour plot analysis for optimal adsorption condition in Cellufine MAX GS resin
Screening for Optimal Adsorption Conditions with Cellufine CEX Resins
Cellufine MAX S-h (dextran modification) and Cellufine MAX GS (graft...
Currently more than 70 biosimilar monoclonal antibodies (MAbs) are under development, and multiple originator MAbs are going off patent in the next three to four years. Protein A affinity media material remains the most important workhorse for the purification of monoclonal antibodies.
Photo 1: Amsphere A3 is a new protein A resin designed with a surfacemodified base bead and alkali-resistant optimized ligand.
Protein A media has a high impact on both development and manufacturing costs, particularly during early stage clinical phases. This note summarizes key performance parameters for JSR Life Science’s Amsphere A3 high-capacity protein A resin for six biosimilar molecules of which five are MAbs, and one is a Fc-fusion protein.
Figure 1: Test conditions of the chromatography process
Material and Methods
Table 1 lists the MAbs and fusion protein used in this study, and Table 2 shows the experimental methods and materials. Figure 1 outlines the steps of the chromatography process and the test conditions. ...
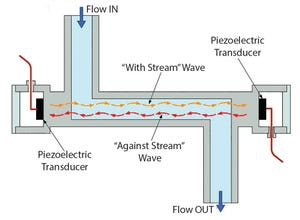
Levitronix® has released the first ultrasonic, single-use (SU) flowmeter on the market. Characterized by high accuracy and superior performance, LEVIFLOW® SU noninvasive flowmeters enable a highly precise and low-cost flow measurement. With an accuracy of <1% of reading and flow ranges from 1 mL/min to 80 L/min, they offer the highest level of accuracy for measuring liquids in biopharmaceutical and other applications that require FDA, USP-VI, BSE/TSE, and animal-component–free materials that can be gamma sterilized.
Figure 1: Operating principle of ultrasonic single-use sensor
Technical Background:
Figure 1 illustrates the operating principle: Two piezo-electric transducers, mounted in the sensor housing, generate and receive an ultrasonic wave. The wave going in direction of the flow (with-steam wave) is accelerated, and the wave going against the flow direction (against-steam wave) is slowed down. Both waves are processed by a signal converter. The difference of the transit time of both waves is propor...
The new LEWA EcoPrime® LPLC chromatography and enhanced buffer dilution platform combines proprietary fluid design and the LEWA Intellidrive® pump technology to deliver unrivaled reproducibility and accuracy. The integrated buffer in-line dilution (BID) option prepares point-of-use buffers combining two unit operations into a single, space-saving skid.
A single LEWA EcoPrime system has the flow range of up to two conventional LPLC units while delivering gradient accuracy of greater than ±1%. Because of its dynamic range, EcoPrime LPLC can bridge the gap between process development and pilot and production scales. The platform is highly configurable with 20 standard options to match your individual situation: full drainability, clean-in-place (CIP) enhancement, multiple inlet/outlet valve choices, precolumn analytics, DeltaV communication, and UPS ready are a few of the many user-configurable options. The EcoPrime LPLC series of systems provides significant flexibility, more floor space and — as a result o...
Bioprocess intensification leads to more efficient, flexible, and cost-effective biopharmaceutical manufacturing facilities. Single-use systems designed for continuous operation facilitate process intensification.
Pall Life Science’s Cadence™ BioSMB PD platform is the first disposable flow path, continuous multicolumn chromatography solution that is fully scalable from a process development laboratory to good manufacturing practice (GMP) manufacturing. It provides an economically viable, disposable chromatographic solution for even the most demanding and costly chromatographic steps.
Figure 1: Principles of simulated-moving bed technology
During multicolumn countercurrent (also known as simulated moving bed, or SMB) chromatography, all chromatographic steps normally run in a batch mode are conducted simultaneously in different “zones” (Figure 1). Chromatographic media reaches full lifetime within a campaign through rapid cycling, making disposable use of even the most expensive chromatographic media possi...
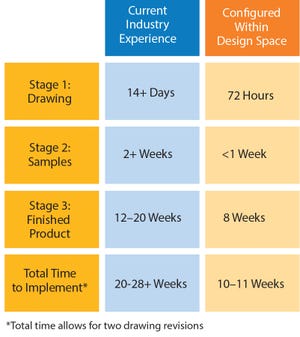
In June 2016, Parker domnick hunter began manufacturing in its new single-use facility in Birtley, Co Durham, UK. Here I discuss the approach taken in the design, testing, and manufacturing of single-use assemblies and some of the advantages they can have in terms of lead times, inventory control, and supply chain robustness.
Challenges of Customization
For some time, the message relayed to biopharmaceutical manufacturers was to design exactly what you want and need for each process. That meant a customized solution for every operation, for every scale, and for every product. There is no doubt that single-use technology has dramatically increased the flexibility of biopharmaceutical manufacturing facilities, but this has led to some challenges for the implementation of single-use systems.
High levels of customization can mean that organizations have to stock and control a large number of low-volume items, including managing and (where necessary) writing off expired stock. In addition, the requirement for ...
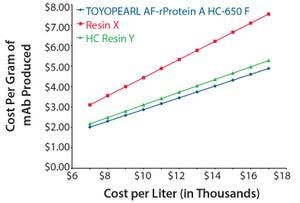
TOYOPEARL AF-rProtein A HC-650F and two other commercially available protein A resins (one of them also a “high capacity” resin) were compared on a cost-per-gram of monoclonal antibody (mAb) produced basis at multiple resin prices from $7,000 to $17,000 per liter as well as three different column configurations. For in-depth comparison, the median resin price of $12,000 per liter was used as a basis to determine comparative production costs among the three resins tested. The configuration used to model what the resin costs would be per gram purified was a column that was packed to have equal column dimensions of 36 cm ID × 15 cm.
Figure 1: Columns of equal dimensions
For this evaluation, the following values were held constant: residence time = 3 minutes; dynamic binding capacity (DBC) = 3 minute residence time (from product literature); column load = 80% of stated DBC; harvest titer = 3 g/L; harvest volume = 2,000 L; column lifetime = 100 cycles; column yield = 95%.
As shown in Figure 1, at a column capa...
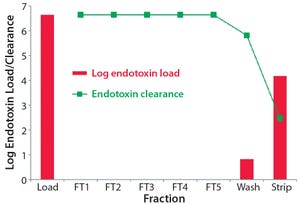
Various unit operations are needed in downstream processing (DSP) of biologics to remove process‑related impurities such as host‑cell proteins, nucleic acids, viruses, and endotoxins. This application note describes the potential of a salt‑tolerant anion‑exchange resin to achieve outstanding clearance of endotoxins and viruses.
Introduction
Endotoxins are lipopolysaccharides (LPS) originating from cell membranes of Gram‑negative bacteria. They are frequent contaminants of recombinant proteins produced in microbial expression systems. On the other hand, expression of more complex proteins in eukaryotic systems carries an inherent risk of introducing adventitious viruses.
TOYOPEARL NH2‑750F, a salt‑tolerant anion‑exchange resin, is ideal for intermediate purification of monoclonal antibodies (mAbs) and other proteins. Aggregates and negatively charged impurities, such as DNA, viruses, and endotoxins can be removed from a target of interest.
Figure 1: Log endotoxin clearance for each step and the correspondi...
Sponsored Content
Alcami specializes in all phases of pharmaceutical development — from critical preformulation studies to commercial product life-cycle management. Over the past 30 years, Alcami has supported more than 500 investigational new drug (IND) filings and over 50 new drug applications (NDA), abbreviated new drug applications (ANDA), and new animal drug applications (NADA). Our fully integrated, comprehensive development services are well established and ready to help our clients get the most out of their portfolios.
Principal Offerings
Drug Development
Alcami’s
analytical services
ensures that robust methods can be transferred throughout the world:
Alcami’s
formulation services
have proven know-how and timeliness for every development project:
Manufacturing and Packaging
API Manufacturing
Drug Product — Oral Solid Dose
Drug Products — Parenteral
Packaging Services
Support Services
Our in-house expertise in regulatory affairs and quality assurance can support your projects, inspections, and filings:
Catherine...
Sponsored Content
Associates of Cape Cod,® Inc. Contract Test Service (CTS) laboratory specializes in method development, bacterial endotoxin testing (BET), and glucan contamination. With the most extensive experience of any endotoxin testing laboratory in the world, CTS is GMP compliant, ISO registered, and licensed by the US Drug Enforcement Agency (DEA) as a laboratory capable of handling all controlled drug substances except those included in Schedule I. Endotoxin testing can be performed in accordance with US Food and Drug Administration (FDA), United States Pharmacopeia (USP), European Pharmacopoeia (EP), and/or Japanese Pharmacopoeia (JP), depending on your specifications.
In addition to routine testing, CTS has extensive expertise and the ability to customize endotoxin testing to individual client needs, develop methods for difficult samples and devices, transfer BET test methods, and design and produce custom depyrogenation controls for oven validations.
CTS has experience with the following
sample types
:
CTS qu...
Avid Bioservices is a contract development and manufacturing organization (CDMO) specializing in mammalian cell culture process development and CGMP production of clinical and commercial-scale monoclonal antibodies, recombinant proteins, and enzymes. Committed to the success of our clients, our team constantly strives to build partnerships that extend well beyond the delivery of your product. By combining our knowledge and experience with a flexible and efficient approach, we can meet your specific project requirements
Established CDMO with Proven Capabilities
Thought Leadership in Process Sciences
Avid’s expanded process sciences group is focused on the ongoing development and enhancement of process and analytical methods needed to deliver optimized processes for transfer to CGMP manufacturing. Our experience with CHO-based antibody production and our knowledge of recombinant protein and enzyme process development provide the expertise you need to advance your programs to the next phase:
New State-of-the...
From drug and biologic development to delivery technologies and supply solutions, Catalent is the catalyst for your success. With 80+ years of experience, we have the deepest expertise, broadest offerings, and the most innovative technologies to help you get more molecules to market faster, enhance product performance, and provide superior, reliable manufacturing and improved results. Catalent is your strategic partner for biologic drug development and manufacturing success.
SMARTag™ technology
offers a new toolkit to optimize antibody–drug conjugates (ADCs) and bioconjugates. It allows site-specific, programmable drug placement using proprietary cytotoxin-linkers and conjugation chemistry in an efficient and scalable process. The result is improved ADC consistency, superior linker stability, and compatibility with any cell-based expression system.
Biomanufacturing Center of Excellence — Increasing Flexibility and Manufacturing Scale for Customized Solutions:
Our state-of-the-art biologics manufacturing...
byMin Park
Expertise
Cobra Biologics is a rapidly expanding international contract development and manufacturing organization (CDMO) of biologics and pharmaceuticals for clinical and commercial supply. With three GMP-approved facilities, each with expertise tailored to serving our customers around the world, we offer a broad range of integrated and stand-alone contract services stretching from cell line and process development through to fill and finish for the supply of investigational medicinal products and commercial production.
Experience
For over 18 years we have taken pride in being a trusted manufacturing provider, delivering what we promise and helping our customers to develop medicines for the benefit of patients. Cobra provides manufacturing technologies, platforms, and solutions for the pharmaceutical industry covering antibodies, recombinant proteins, viruses, phage, DNA as gene therapies, and whole-cell vaccines and therapeutics, as well a...
Ensuring optimal and maximal T-cell production is critical for adoptive immunotherapy and its continued success. The Xuri Cell Expansion System is an important component of the clinical manufacturing process. So we sought to investigate the effect of rocking rate and angle on the expansion of T cells. We used a combination of experimental data and predictive modeling and found that the rocking rate significantly influences the expansion potential of T cells with minimal contribution from the rocking angle. The results indicate that a rocking rate of 15 rocks per minute (rpm) and an angle of 6° are optimal for a 1-L bioreactor to maximize cell growth using a Xuri Cell Expansion System.
Introduction
The rapid and reproducible expansion of highly specific T cells from low precursor frequencies to clinically relevant numbers is essential for the success of autologous cell therapy techniques. The Xuri Cell Expansion System uses media perfusion and a rocking platform to achieve cell densities >10 × 10
6
/mL i...
bySam Kale