Richter-Helm is a Hamburg, Germany–based contract manufacturing company with a proven 30-year track record and specialized in products derived from bacteria and yeasts. Count on us to flexibly provide a comprehensive range of services and customized solutions. Clients worldwide already have benefited from our commitment to good manufacturing practice (GMP) and total transparency. Our work focuses on recombinant proteins, plasmid DNA, antibody fragments, and vaccines.
Our seasoned, 240-strong team supports you with process development, supply of products for clinical trials, commercial production, in-house quality control (QC) testing, and QP release. We operate two GMP-compliant production plants with bioreactor capacities of up to 1,500 L.
Richter-Helm consistently works to the highest standards of pharmaceutical quality, as verified by major regulatory bodies, including the European Medicines Agency (EMA), the US Food and Drug Administration (US FDA), Agência Nacional de Vigilância Sanitária (ANVISA), a...
Nordson MEDICAL has launched a new manifold that makes assembly and operation simpler compared with products currently used in the biopharmaceutical industry. This patent-pending product was announced at ACHEMA in Frankfurt, Germany, in 2018. Simplicity is the main benefit of the design and user interface built into CYLINDRAFlow™ manifolds. There is a one-to-one relationship between the inlet port and the five available outlet ports. The large, easy-to-use knob with clearly marked arrows provides a visual indication of which outlet port is in use, and once properly positioned, the handle locks in place, assuring an operator that the flow path is set up correctly and that it can’t accidentally change.
Just fill out this form to read the full PDF and learn more.
The field of cell and gene therapy is transforming the way patients who are diagnosed with cancers or genetic diseases can be treated. Today, the cost of producing these therapies still represents a major hurdle on the way to the commercialization. New technologies are needed that enable robust and cost-efficient manufacturing and yield replicable high-quality medicines. Although the therapeutic opportunities for patients are exciting, the stakes for patients and drug developers are high.
We want to be your partner, add value to your therapy development process, and drive pioneering therapies to the market. We invest in enabling technologies and build expertise to support the development and commercialization of new innovative therapies. Our scientists and engineers bring decade-long development experience across a broad spectrum of cell types and technologies. That expertise builds the backbone of an extensive service offering, providing you with tailored process and analytical development, manufacturing...
Wacker Biotech is “The Microbial CMO” — the partner of choice for process development and contract manufacturing of biopharmaceuticals (proteins, vaccines, and live microbial products) using microbial hosts. WACKER’s integrated service portfolio covers molecular biology, process/analytical development, and good manufacturing practice (GMP) manufacturing of biologics for clinical and commercial supply.
WACKER operates three state-of-the-art GMP facilities located in Europe (Germany and The Netherlands). Manufacturing lines with two 300-L and two 1,500-L stainless steel fermentation vessels, single-use fermentation lines, matching downstream processing scales and fill and finish capabilities are available to suit all possible customers’ needs (BSL 1 and 2).
WACKER holds biomanufacturing certificates from the relevant authorities for all sites and follows the ICH Q7A guidelines for GMP-compliant production of active pharmaceutical ingredients (APIs) and drug products (DPs). All three GMP production facilitie...
Three-dimensional (3D) culture systems provide cell–cell and cell–extracellular interactions that reproduce the cellular microenvironment in vivo better than typical two-dimensional monolayers. This property is of paramount importance in many applications, including disease modeling, drug toxicity assessment, and manufacturing of stem-cell–based products (
1
). Cultivation in stirred-tank bioreactors using perfusion mode opens up new possibilities in the cultivation of 3D cell aggregates.
Perfusion allows for removal of detrimental metabolites, cell debris, and proteases from the culture as well as the addition of fresh nutrients. That facilitates longer cultivation periods than are achievable with traditional batch systems (
2
) and allows for establishing concentration gradients and smooth transitions between growth factor concentrations in a culture medium. Such capabilities are particularly relevant for bioprocessing stem cells or 3D cell models because they can provide a more physiologically accurate...
Biopharmaceutical companies are striving to produce biologics more efficiently with a lower cost point and fewer employees. Most small-scale media and buffer operations are carried out with small bottles of liquid media. But as processes are scaled up to manufacturing levels, that is no longer a viable option. Costs of shipping liquid are simply prohibitive, so the industry has migrated to using media and buffer in powder form. In fact, 90% of cell-culture media and buffer for sale is now in powder form, which needs to be hydrated, mixed, and packaged to be used in bioprocesses. This “just in time” hydration allows for increased compliance and process efficiency.
Making biologics safely also means designing a process to reduce contamination risk at every step, even where it may not seem obvious to do so. One often overlooked step is mixing media and buffer powder during a process. It is still common to see people pouring open buckets of powdered media into a mixing tank, with powder flying into the air an...
Chinese hamster ovary (CHO) cells are used widely in the biopharmaceutical industry for the production of recombinant proteins. In times of rapid growth in the biopharmaceutical market with monoclonal antibodies (MAbs) and biosimilars, the use of chemically defined (CD) feeds and culture media for CHO mammalian cell culture is crucial. Currently, strict regulatory norms for the biopharmaceutical industry has led to the wide use of animal-component–free (ACF) culture processes, including CD media and feeds. A business requires significant amounts of time and investment to develop an optimal CD fed-batch medium solution that fits the needs of both the company and its process. Many companies might not have the resources to develop their own CD media.
Kerry now offers two CD media for extended growth and enhanced recombinant protein production for CHO suspension cultures. AmpliCHO CD medium is a protein-free, serum-free medium that has been optimized so that it can be seamlessly combined with Kerry’s individu...
Complex proteins present a range of production challenges, including low expression levels, degradation, aggregation, a high degree of posttranslational modifications, and the need to use multiple or large expression plasmids. They also can lead to challenges in producing multi-subunit proteins with correct folding and subunit ratios.
MaxCyte’s ExPERT platform for cell engineering alleviates those challenges by facilitating the production of complex proteins with high efficiency and cell viability in your cell line of choice. This technical note summarizes four applications illustrating the range, efficiency, and effectiveness of MaxCyte’s flow electroporation–based delivery platform.
Just fill out the form to read the full PDF and learn more.
Built on over 30 years of expertise in large-volume fluid processing with 3D single-use bags, Sartorius Stedim Biotech collaborated with many end users for the large-volume Flexsafe® 3D system with enhanced ergonomics, thereby bringing operator safety to the center of successful drug processing.
We conducted in-depth interviews to gather and explore operator insights into the usability of large-volume single-use systems. That input helped us improve our product offering with the aim of also improving workplace health and well being. A focus on ergonomics in single-use operations is a key factor toward achieving easy, fast, and safe liquid handling. A decrease in the appearance of musculoskeletal symptoms in workers can provide a better workplace, with positive effects on worker satisfaction, product quality, and process efficiency.
The large-volume Flexsafe® 3D system is designed specifically for storage of large-volume process fluids up to 3,000 L. Applications include
Just fill out the form below to rea...
CIMmultus ADC monoliths from BIA Separations are the first chromatography products in the field to exploit hydrogen bonding as the primary binding mechanism. These products bring a unique new selectivity to all purification challenges, but they are especially distinctive in their ability to retain large biomolecules more strongly than small ones. They enhance removal of fragments, aggregates, and viruses from proteins, and they enhance removal of proteins and other small contaminants from viruses and other very large biologics. H-Bond ADC also supports outstanding DNA removal.
Hydrogen bonds are polar, and they can be dissociated with salts, but they require higher concentrations than ion exchangers. This makes H-Bond ADC more tolerant of salt in applied samples. Most samples can be applied at pH 7.0 in 100 mM NaCl. Large biologics often can be applied in 200–300 mM NaCl. Salt tolerance increases at lower pH values. This simplifies sample preparation and minimizes the need to dilute or buffer-exchange sam...
Appropriate handling and removal of nucleic acids is crucial for many applications in red biotechnology and molecular biology. Upon the production of many biopharmaceuticals in fermentative processes (e.g., proteins, antibodies, and vaccines), nucleic acids are concomitantly generated and accumulated in culture broth, particularly when microbial cells are disrupted during work-up. Such accumulation increases the viscosity of the liquid fraction significantly and complicates downstream processing and purification. To be marketable and comply with strict safety regulations, biopharmaceutical products need to be nearly free of nucleic acids. Therefore, a nucleic acid removal step is mandatory before commercialization of (fermented) biopharmaceuticals is considered.
How can nucleic acids of a biological sample be removed without degrading a marketable product? Some traditional approaches include sonication, precipitation, and extraction strategies, which have been demonstrated in different applications dealin...
Failure is not an option. When a single drop of fluid can make a huge difference in your single-use system, you need smart, reliable, and easy-to-use fluid handling solutions that get the job done right and safely. That is why biopharmaceutical manufacturers from all over the world rely on CPC’s AseptiQuik® connectors. They provide quick and easy sterile connections, even in nonsterile environments. They feature an easy-to-use three-step assembly and come in a wide range of size options and genderless connections. These sterile connectors allow you to transfer media easily with less risk of operator error than with other connectors.
As the industry grows, a demand for higher flow rates has developed. CPC is at the forefront of fluid handling innovation, and we’ve taken our AseptiQuik connectors up a notch — up to 1 inch, that is. We’re proud to introduce our newest and largest connector: the genderless AseptiQuik 1-inch large-format connector. This newest addition to the CPC catalog extends the range of g...
A new product development approach will be described here for the affinity capture of monoclonal antibodies (MAbs) from cell culture materials using a novel base stable rProtein A ligand with up to six available Fc binding sites. The resin is based on a stable cellulose bead structure with excellent flow properties combined with the affinity ligand immobilized at multiple sites to yield a robust next generation MAb capture resin with a high level of binding capacity.
These resins have been developed to retain >95% of their original binding capacity after >100 cycles of reuse under 0.1M NaOH clean-in-place (CIP) conditions. Polyclonal and MAbs show efficient elution at pH 3.5 with a 0.1M glycine HCL or 60 mM acetic acid buffers. This new Cellufine Protein A suite of resins developed by JNC will offer flexibility for future continuous workflow formats as well as being compatible with existing chromatography systems.
Just fill out the form to view the full PDF and learn more.
The LEWA EcoPrime® Twin LPLC multicolumn chromatography system is easy to use and GMP ready. It offers seamless scale-up for continuous batch chromatography from process development to large-scale biopharmaceutical production. The EcoPrime Twin platform spans a wide range of flow and column sizes and has several options to incorporate multiple functionality on a single unit. The EcoPrime Twin system is built on ChromaCon’s Contichrom patented approach to twin-column purification. Because of the two-column configuration, the EcoPrime Twin is a simple design that facilitates continuous chromatography implementation, reduces risk of costly downtime, and minimizes operating expenses. It is well suited for capture steps using protein A or ion-exchange (IEX) separations for monoclonal antibody, recombinant, or plasma purification processes.
Just fill out this form to read the full PDF and learn more.
Until now, one chromatography system was needed to execute one chromatography step. With one three-module BioSC® system, three chromatography separations — including viral inactivation (VI) and in-line dilution (ILD) — can be operated simultaneously and continuously. This system is a straightforward switch from several batch skids to a single DSP skid (Figure 1).
One skid performing an entire downstream process from capture to polishing can reduce a facility footprint drastically and decrease costs significantly. One key benefit of using this system is the elimination of steps that do not add value (e.g., intermediate storage), human intervention (safety, batch failure), and equipment such as holding tanks.
Just fill out the form below to view the full PDF and learn more.
Optek-Danulat GmbH has been providing solutions for the bioprocessing industry for over 30 years. optek inline analyzers measure turbidity, UV-Vis-NIR absorbance, color, pH, and conductivity using innovative technologies for accurate and reliable results.
The Single-Use Cell System for Downstream Bioprocessing:
optek’s single-use cell (S.U.C.) system consists of the disposable cell, a stainless-steel cell holder, proven optical sensors also used in conventional systems, and a converter capable of operating optical, pH, and conductivity sensors. The system is designed to optimize separation, purification, and concentration processes in disposable chromatography and ultrafiltration systems. The single-use approach offers several significant advantages over conventional stainless-steel systems. Single-use systems eliminate the need for cleaning and validation and reduce cross contamination risks, leading to reduced down time between batches and improved productivity.
Just fill out the form below to read the...
The adoption of disposables or single-use technology (SUT) in biopharmaceutical manufacturing is growing rapidly. SUT offers improved flexibility, reduced risk of contamination, and lower overall costs. Bags made from laminated polymers are used widely as disposable containers for the freezing and storage of bulk drug substance (BDS). But if the properties of a BDS require it to be stored at –70 °C, then fluoropolymer (perfluoroalkoxy (PFA) or fluorinated ethylene propylene (FEP)) bottles are the only choice because those materials have much wider working temperature ranges. And although SUT bags have been designed for biopharmaceutical manufacturing, the only fluoropolymer bottles available until recently were designed originally for use in the chemical industry.
Savillex’s Purillex™ PFA and FEP bottles are the first fluoropolymer bottles designed specifically for the biopharmaceutical industry.
Just fill out the form below to view the full PDF and learn more.
Antibody–drug conjugates (ADCs) are promising biopharmaceuticals. They combine the high selectivity and affinity to cancer cells with the toxicity of chemotherapeutics in one molecule. ADCs consist of a monoclonal antibody covalently bound by a linker to a highly potent cytotoxic drug. ADC-surrogates contain a nontoxic payload with similar structure and physiochemical properties as the toxic payload of an ADC. Therefore, they can be used as models to develop suitable purification processes or analytical methods. The ADC-surrogate in this work consists of adalimumab bound to fluorescein 5-isocyanate (FITC).
The purification process of ADCs is complex because of the heterogeneity of the conjugates. One of the main challenges is the separation in different drug–antibody ratios (DARs), which correlate with the potency of the ADC. High DARs are associated with high cytotoxic levels whereas low DARs affect the efficacy of therapeutics. Because of the very hydrophobic payload, a higher DAR results in a greater h...
Avid Bioservices is a full-service, dedicated contract development and manufacturing organization (CDMO) focused on development and manufacturing of biopharmaceutical products derived from mammalian cell culture. Avid provides process development and CGMP clinical and commercial product manufacturing and offers expertise supporting analytical development, qualification, and validation activities. Additional service offerings include cell line optimization, cell culture and feed optimization, product characterization, and stability testing. Avid has 25 years of experience producing a comprehensive range of proteins, including monoclonal antibodies and recombinant proteins in batch, fed-batch, and perfusion modes as well as bispecific monoclonal antibodies, Fc-fusion proteins, and biosimilars.
Just fill out this form to view the full PDF and learn more.
Sponsored Content
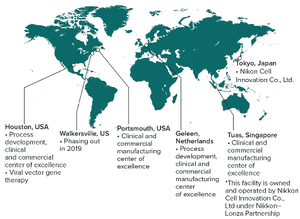
Developing a long-term manufacturing-ready process for gene therapy and viral vaccine products can be a challenge for a number of reasons. First-in-human trials require developing a process with limited knowledge, and success metrics are unknown (e.g., assays to measure success have not yet been developed). In addition, the components of a treatment are difficult to buy or make, and in many cases, components of a process are rare and exist in very small quantities. Finally, such a process is difficult to scale up because current manufacturing processes may not be not amenable to scale up.
Additional challenges can be found early in the development stage, including selecting the right combination of host/virus systems. Selecting the right combination will depend mainly on safety, toxicity, and the ability of a host/virus combination to effectively produce a target product.
At Fujifilm Diosynth Biotechnologies, process and analytical development, technical evaluation, selection, and ordering of process equi...
Sponsored Content
Capsule Delivery Solutions, part of Lonza Pharma and Biotech, is the leader in capsule-based solutions and services, proudly offering Capsugel® products. With the largest production and supply chain footprint in the industry, Capsule Delivery Solutions provides the highest quality and deepest regulatory expertise to its 2,000 pharmaceutical customers, globally.
For more information, email
[email protected]
, visit
www.capsugel.com
and follow us on Twitter, LinkedIn, and YouTube.
Just fill out the form to view a full PDF and learn more.
Pfizer CentreOne is a global contract development and manufacturing organization (CDMO) embedded within Pfizer. We focus on custom API synthesis, sterile injectable fill–finish, and highly potent oral solid dose; and we are a leading supplier of steroid and hormone APIs and intermediates. For more than 40 years, we’ve manufactured complex compounds for our biopharmaceutical partners, guiding their drugs safely from development through commercialization.
The Value of the Embedded-CDMO Model: Your molecule is manufactured in the same facilities that produce Pfizer’s own drugs, with the same vigilance and care. Throughout the development process, you’ll work with a dedicated program manager and core team of experts who deeply understand your molecule and business goals. Should challenges arise that require specialized knowledge, labs or technology, our experts can harness literally a world of Pfizer resources on your behalf, while serving as your advocates across the broader Pfizer organization.
Just fill ou...
For over 25 years, Prometic Bioseparations Ltd (PBL) has produced chromatography adsorbents in batch sizes up to 280 L under cleanroom/good manufacturing practice (GMP) conditions at its Isle of Man (UK) manufacturing site. Following a sustained increase in demand for its chromatography products, PBL made the decision to expand its manufacturing operations to meet the projected future demand. During 2013, designs to expand the plant were developed. Between 2014 and 2017, a major investment focused on the expansion of PBL’s Isle of Man manufacturing site to provide a significant increase in production scale. Maximum batch sizes have increased from 280 L to 1,000 L, and manufacturing capacity has increased to approaching 50,000 L per year. This makes Prometic one of the largest manufacturers of affinity adsorbents in the world.
At the heart of the new production capacity are 2 × 2,500-L reactors and associated buffer tanks and plant equipment, which enable GMP manufacture of individual adsorbent batches up ...
Rentschler Biopharma SE — located in Laupheim, Germany — is a leading contract development and manufacturing organization (CDMO) focused exclusively on client projects. The company’s clients include innovative biotech companies and major pharmaceutical companies around the world. Rentschler Biopharma has long-standing experience and proven excellence as a solution partner of choice. A top-notch quality management system, well-established operational excellence, and advanced technologies ensure product quality at each development and manufacturing step. Rentschler Biopharma is a family-owned company with over 850 employees.
One-Stop Solutions from Gene to Vial and Clinic to Market:
Rentschler Biopharma’s end-to-end offering includes biopharmaceutical process development and manufacturing as well as related consulting activities, including project management and regulatory support. With a focus on mammalian cell lines, Rentschler Biopharma is highly experienced in the development and CGMP manufacturing of ...
Environmental monitoring, product quality control, and mycoplasma testing of biopharmaceuticals require anaerobic bacteria monitoring. If the proper environment is not created when performing microbial testing, bacteria will not grow, and a possible contamination could be missed. Any bacterial contamination of biopharmaceutical products can have major negative effects on manufacturing, production costs, and profit margins.
A technology that relies on the evacuation and replacement of oxygen in a jar is valuable to the biopharmaceutical industry because it creates a quality-assured anaerobic environment within minutes. The Anoxomat system helps create anaerobic and custom environments that promote bacterial growth, essential to controlling the quality of biomanufacturing.
Just fill out this form to view the full PDF of this white paper.
Chinese hamster ovary (CHO) cells are the expression system of choice for the manufacturing of biotherapeutics. They have the capacity to express a range of proteins such as therapeutic enzymes or monoclonal antibodies at multiple gram per liter titers.
As expression technologies have developed, obtaining such titers has been achieved mainly through improvements to media and feed. Similarly, the ability to identify high-productivity clones has been streamlined through the use of different selection systems, with metabolic selection being the preferred method in the bioprocess industry.
…Horizon was able to use its strong IP position to generate a CHO K1 GS knockout cell line with clear freedom to operate in biomanufacturing. Industry adoption has grown significantly with over 20 full commercial licenses currently in place and a strong pipeline of new licenses pending…
Just fill out this form to view the full PDF of this white paper.
Early stage development of biological unit operations is critical to ensuring an efficient and robust commercially viable process. Often, the types of experiments required in early stage development are arduous tasks that require extensive testing of different critical process parameters, filters, and other essential production components. Such testing inherently produces massive amounts of data that must be carefully analyzed. To streamline the development process and optimize operational efficiency, PendoTECH has developed a full offering of control systems specifically designed for the development of purification processes. These control systems start with offerings for filter screening and benchtop experiments and continue all the way up to pilot-plant production environments.
Just fill out this form to view the full PDF of this white paper.
As an engineering company, we are optimizing and developing new technologies for more efficiency in bioprocessing. Building on over 25 years of experience in online analytics, information processing, and automation technology, we aim to be the trusted technology provider for optimized workflows through automated sampling and process information management.
Who We Are
Securecell emerged in 2009 from the company Biospectra, which was founded as a spin-off from ETH Zürich in 1992. Ever since, development of online analytic solutions for bioprocess applications has been one of our focus areas. Our urge to deliver the best solutions for our customers’ specific needs makes us relentless in optimizing and developing new technologies for more efficiency in bioprocessing.
Fill out the form below to read the full PDF of the white paper.
IgM antibodies are one of the fastest growing groups of diagnostic and therapeutic candidates. Unlike IgGs, IgMs cannot be purified by affinity chromatography due to their physiochemical properties. The large size of IgMs presents an additional purification challenge. Bio-Rad has developed a new strong anion exchange (AEX) resin — Nuvia HP-Q — to overcome the multiple issues faced when purifying large biomolecules. We show the purification of an acidic IgM antibody with capture purification using Nuvia HP-Q and polish purification using mixed-mode CHT Ceramic Hydroxyapatite Media. In initial experiments, the capture step removed process-related impurities while polishing with ceramic hydroxyapatite depleted these impurities further. The described purification strategy is simple and scalable. The studies resulted in product purity >99% IgM, as measured by size exclusion chromatography and SDS-PAGE analysis. This purity makes the IgM suitable for diagnostic applications.
Just fill out this form to view the ...