A high-throughput method for relative screening of terminal sialic acid content was developed on the Octet platform to expedite cell line development. The method is based on the use of ForteBio’s sialic acid (GlyS) kit to bind sialic acid on glycoproteins. The GlyS kit can screen specifically the sialylation levels of secreted proteins in crude cell culture samples and does not require purified samples. Using this method, 96 crude cell culture samples can be screened for sialylation in 60 minutes or less. Here we used the GlyS kit to determine the relative sialylation level of representative glycoproteins, including erythropoietin (EPO), standard human monoclonal antibody (MAb) samples (NIST MAb), and fetuin glycoproteins, as well as crude biosimilar cell culture samples using an Octet HTX system. In a comparison study of the Octet GlyS kit method and high-performance liquid chromatography (HPLC), a linear correlation (R > 0.9) was observed between the GlyS kit binding signal (nm/g) obtained using an Octe...

 +4
+4The natural characteristics of Chinese hamster ovary (CHO) cells have contributed to their use as the dominant expression platform in biomanufacturing. To increase the capabilities of these host systems, fundamental improvements to the CHO cell line are needed, which is where we believe that gene editing can offer a step change in biopharmaceutical manufacturing.
Fill out the form below to read the complete technology review now.
Adeno-associated viruses (AAVs) are the most commonly used type of viral vector applied in gene therapy trials to date (
1
). From a regulatory perspective, an understanding of the critical quality attributes (CQAs) that impact product safety, purity, and potency is required. Specific analytical assays to assess vector productivity, vector purity, biological activity, and safety are required to generate data to ensure that the vector is produced with the consistency necessary to ensure safety and prevent undesired affects (
2
) on patients’ health while maximizing clinical benefit.
Fill out the form below to read the complete technology review now.
References
1
Ginn SL, et al. Gene Therapy Clinical Trials Worldwide to 2017: An Update.
J. Gene Med
. 20, 2018: e3015.
2
Chemistry, Manufacturing, and Control Information for Human Gene Therapy Investigational New Drug Applications, Draft Guidance for Industry
. Food and Drug Administration: Silver Spring, MD, 2018.
An extensive suite of contract testing and development services combines Sartorius Stedim Biotech’s expertise in cell line development, bioanalytical testing, and biosafety contract testing to support customers from cell line development through to product manufacture and release. Broadening our portfolio of products and technologies, these services enable a comprehensive “total solution” to support our clients’ drug development activities.
Benefit from our extensive experience to efficiently advance your drug candidates quickly from early stage development through to commercialization. Based on unparalleled global experience working to support biological drug development, Sartorius Stedim Biotech leads the way in preclinical development. Through robust
platforms, off-the-shelf assays, and commitment to providing a total solution, we can accelerate your drug’s development.
Fill out the form below to read the complete capabilities review now.
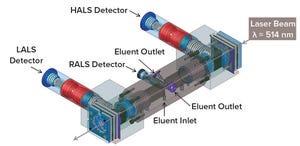
The multi-angle light scattering (MALS) technique has become the standard for determining molecular weight (MW) and size (radius of gyration, Rg) of proteins, biopolymers, synthetic polymers, and polysaccharides. By combining an extreme low angle (low-angle light scattering (LALS) – 10°) and an extreme high angle (high-angle light scattering (HALS) – 170°) with a right angle (right-angle light scattering (RALS) – 90°) to form a three-angle MALS detector, the new LenS
3
detector can determine directly absolute molecular weight and Rg without extrapolation, unlike other detectors already available.
By contrast with conventional flow cells, the new extended “flow chamber” allows maximum interaction of the incident beam with the molecules of interest, resulting
in significantly higher scattering intensity. Coupling this unique design feature with a low laser wavelength of 514 nm (instead of the typical 660-nm wavelength), the LenS
3
detector offers detection sensitivity unmatched by any other instrument. Un...