Richter-Helm is a Hamburg, Germany–based contract manufacturing company with a proven 30-year track record and specialized in products derived from bacteria and yeasts. Count on us to flexibly provide a comprehensive range of services and customized solutions. Clients worldwide already have benefited from our commitment to good manufacturing practice (GMP) and total transparency. Our work focuses on recombinant proteins, plasmid DNA, antibody fragments, and vaccines.
Our seasoned, 240-strong team supports you with process development, supply of products for clinical trials, commercial production, in-house quality control (QC) testing, and QP release. We operate two GMP-compliant production plants with bioreactor capacities of up to 1,500 L.
Richter-Helm consistently works to the highest standards of pharmaceutical quality, as verified by major regulatory bodies, including the European Medicines Agency (EMA), the US Food and Drug Administration (US FDA), Agência Nacional de Vigilância Sanitária (ANVISA), a...
To enhance their performance, life science specialists require flexibility in their work and tools. The increasing diversity of bioprocess applications calls for different cultivation methods. Therefore, being able to customize processes and equipment is a significant advantage. With over 45 years of experience in customizable multi-use bioreactors, Applikon Biotechnology now provides the same flexibility with customizable single-use
bioreactors.
The AppliFlex ST bioreactor is a fully configurable, stirred-tank, single-use bioreactor that can be designed to specific customer demands. Using three-dimensional
(3D) printing technology, any bioreactor configuration can be created. Important bioreactor features such as impeller design and number and type of liquid and gas additions can be adapted to and optimized for particular cell lines and performance objectives.
Fill out the form below to read the full technology review now.
With the increase of T-cell immunotherapy research, cell therapy developers have encountered the difficulty of producing consistent, high-quality products. Approved therapies Kymriah™ (tisagenlecleucel, Novartis), Yescarta™ (axicabtagene ciloleucel, Kite Pharma), and late-phase clinical products BB2121 and UCART19 have been critiqued for presenting potential obstacles to patient access because of high costs of treatment and variable manufacturing outcomes. Manufacturing processes need to be developed to increase control over each unit step in a way that improves product consistency over the life of a product. Bioreactor culture systems provide a pathway to a higher level of process control over cell expansion.
Fill out the form below to read the complete technology review now.
Substituting traditional glass bioreactors with single-use equipment can simplify a bioprocess workflow. Implementing single-use vessels eliminates the need for cleaning and autoclaving, thus reducing the time needed to prepare a bioprocess run and lowering
contamination risk. Another advantage is that single-use vessels reduce occupational hazards associated with overweight handling (especially at larger bench scales) because plastic weighs less than glass.
Fill out the form below to read the complete white paper now.
Biopharmaceutical companies are striving to produce biologics more efficiently with a lower cost point and fewer employees. Most small-scale media and buffer operations are carried out with small bottles of liquid media. But as processes are scaled up to manufacturing levels, that is no longer a viable option. Costs of shipping liquid are simply prohibitive, so the industry has migrated to using media and buffers in powder form. In fact, 90% of cell culture media and buffers for sale is now in powder form, which needs to be hydrated, mixed, and packaged to be used in bioprocesses. This “just in time” hydration allows for increased compliance and process efficiency.
Fill out the form below to read the complete technology review now.
Virus-like particles (VLPs) have become a promising means for developing vaccines and gene therapies. Currently, vaccines based on VLPs are commercially available for human papillomavirus and hepatitis B and E. Other disease treatments with VLPs are undergoing clinical trials.
Upon expression, the structural matrix polyprotein group-specific antigen (gag polyprotein) from the human immunodeficiency virus (HIV) has shown to accumulate beneath the lipidic membrane. After a sufficient number of gag polyproteins are recruited, the assembly process is finished, and the VLP buds out of the cell. The gag polyprotein is demonstrated to accommodate different protein antigens, underlining their potential as a multivalent vaccine. Several biological systems have been used to produce such nanoparticles, but animal cell lines are the preferred option.
Specifically, the insect cell/baculovirus expression system (BES) has proven to work properly for expression of complex virus proteins, achieving high yields with adequa...
Sartorius Stedim Biotech (SSB), a leading international supplier for the biopharmaceutical industry, has launched the BIOSTAT® RM TX single-use bioreactor, a new wave-action mixer system developed specifically for closed, automated expansion of consistent-quality cell therapies such as ex vivo cellular immunotherapies. This new good manufacturing practice (GMP) platform combines SSB’s established single-use Flexsafe® bag technology with the company’s expertise in biopharmaceutical automation.
Fill out the form below to read the complete technology review.
Expansion of suspension cell culture from cell bank to bioreactor is performed through passages of successively larger Erlenmeyer shake flasks. A traditional cap must be unscrewed for each fluid transfer. Risk of contamination is mitigated by performing these fluid transfers in a biosafety cabinet (BSC) or laminar flow hood. Work in a BSC is not preferred because of high maintenance and operating costs, intensive cleaning and decontamination procedures, and the inconvenience of performing operations in the BSC. Despite the use of a BSC, cultures are split to create back up flasks to be used in case of a contamination. Cell expansion can be improved by meeting the following objectives:
Problem Statement
Cellular respiration consumes O and produces CO
2
as a byproduct. Cultures with high CO
2
levels become acidic and impair cell viability. Improper O
2
levels can slow or stunt cell growth. Traditional Erlenmeyer flasks have a filter membrane embedded in the cap. The arrangement allows for unrestricted ai...
2D Flexsafe® Bags and Shells Offer Strong Validation:
Fill out the form below to view the complete poster now.
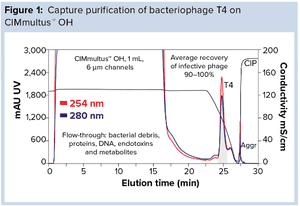
Antibiotics are unable to keep pace with infectious diseases, and the use of bacteriophages is a timely solution to that problem. But implementation of bacteriophages involves overcoming some challenges. Broad-spectrum clinical efficacy will require numerous phages. In turn, cost-effective development of purification procedures will require a platform approach in which one basic process can accommodate all species. That includes the ability to reduce endotoxins to appropriately low concentrations despite the high loads that occur in gram-negative production systems.
Monolithic chromatography media provide a combination
of high capacity, fast flow, and low shear stress to enable
process simplification and purification efficiency that cannot
be reproduced with any other media.
Fill out the form below to read the complete technology review now.
In biopharmaceutical manufacturing, sterility, speed, and equipment ease of use are essential factors. CPC’s AseptiQuik® connectors are specially engineered to provide all three. They create quick and easy sterile connections, even in nonsterile environments. They also allow you to transfer media easily with less risk of operator error than other connectors. All AseptiQuik connectors assemble in an easy-to-use three-step process and come in a wide range of size options and genderless connections.
Fill out the form below to read the complete technology review now.
Cells and cell debris are removed between fermentation (upstream) and product cleaning (downstream) during a process called midstream. Midstream processes often are performed by combining several unit operations (
1
). A highly efficient method is cake filtration, which can be conducted using a FILTRODISC™ BIO SD depth filter. Here, I describe the cleaning of fermentation broths using cake filtration. This technology can maximize product yield and economic efficiency. With increasing particle loads (>108 cells/mL), standard technologies (e.g., centrifugation, separation, and membrane- and depth-filtration) have their limitations.
Fill out the form below to read the complete technology review now.
References
1
Russell E, Wang A, Rathore AS. Harvest of Therapeutic Protein Product.
Process Scale Bioseparations for Biopharmaceutical Industry
. Shukla AA, Etzel MR, Gadam S, Eds. CRC Press, Taylor & Francis Group: Boca Raton, FL, 2007.
Nordson MEDICAL has launched a new manifold that makes assembly and operation simpler compared with products currently used in the biopharmaceutical industry. This patent-pending product was announced at ACHEMA in Frankfurt, Germany, in 2018. Simplicity is the main benefit of the design and user interface built into CYLINDRAFlow™ manifolds. There is a one-to-one relationship between the inlet port and the five available outlet ports. The large, easy-to-use knob with clearly marked arrows provides a visual indication of which outlet port is in use, and once properly positioned, the handle locks in place, assuring an operator that the flow path is set up correctly and that it can’t accidentally change.
Just fill out this form to read the full PDF and learn more.
Pharmaceutical companies take several factors into account when deciding whether to switch from established batch processes to better performing ones, including continuous processing. Such issues include the need to cut cost of goods (CoG), improve product quality consistency, increase process robustness, prevent human error by implementing automation, and so on. However, some challenges remain for some companies integrating these new ways of processing into a current good manufacturing practice (CGMP) environment. One issue is the adaptation of a quality management system. As a drug substance producer and equipment supplier, Novasep knows how quality requirements need to be addressed in this context. We have developed a downstream process (DSP) control strategy to drive a complete, integrated process line on a single skid. Here, you will discover how.
Fill out the form below to read the complete technology review now.
Detailed early-stage development of filtration unit operations is critical to achieving a robust and reliable full-scale process. Despite the importance of this type of development work, fully evaluating a specific unit operation often can be difficult because of time constraints and a lack of material available for experimentation. As a result, a laboratory must have the proper equipment required to conduct efficient, high-throughput experiments. PendoTECH® has developed the ideal tools to address these types of challenges and enable laboratories to quickly and efficiently evaluate a wide range of filters and conditions.
There are two main types of processes these systems work to automate: normal-flow filtration (NFF) and tangential-flow filtration (TFF). Both types of filtration are ubiquitous in biopharmaceutical processing and are used frequently for unit operations such as harvest, virus removal, concentration steps, and buffer exchange. To optimize each step, the proper filter size and filter charac...
Affinity chromatography has a long history of use in biopharmaceutical manufacturing and is a powerful tool in making industrial processes more efficient by reducing the number of steps required to achieve the desired target purity and yield of a final product. It is common, however, for teams to consider affinity chromatography once all other options have been considered, evaluated, and discarded. Such an approach usually translates into a need to develop affinity adsorbents for new targets quickly, efficiently, and economically.
This article outlines the steps required to develop an affinity adsorbent, including the identification of ligands, choice of base matrices, attachment chemistries, use of spacer arms, and other considerations relevant to the development of novel affinity adsorbents for use in biopharmaceutical manufacture.
Fill out the form below to read the complete technology review now.
Praesto® Jetted A50 is the first protein A resin to meet the future demands in monoclonal antibody (MAb) processing today. It delivers enhanced performance characteristics through Purolite’s patented manufacturing technology: Jetting. This continuous manufacturing process — combined with a novel, alkaline-stable continuous manufacturing process with a novel, alkaline-stable protein A ligand called NGL Impact A™ (supplied by Repligen Corporation) — results in the only bioprocess-scale agarose available with a uniform particle-size distribution. Compared with other resins, Praesto®
Jetted A50 resin delivers superior performance characteristics such as:
Fill out the form below to read the complete technology review now.
Fluoropolymers play a critical role in the storage, freezing, and shipping of critical bioprocess fluids. Not all fluoropolymers are ideally suited for all applications, so it is important to understand the characteristics of each and the benefit of using one over another for your specific application.
Most Common Fluoropolymer Materials
Fluoropolymers have more commonalities than differences. Such characteristics include:
Fill out the form below to read the complete technology review now.
With their integrated electronics board, Sonoflow and Sonocheck sensors offer the smallest equipment footprint solution on the market. By using SONOTEC’s reliable and reusable sensors, you can improve process stability, achieve easy data transfer, and save costs associated with disposables. With the aim of implementing flexible production and minimizing contamination risk, a number of applications in the biotechnology industry need accurate flow measurement and bubble detection.
Fill out the form below to read the complete technology review now.
Sponsored Content
Ajinomoto Bio-Pharma Services is a fully integrated contract development and manufacturing organization (CDMO), with sites in Wetteren and Balen (Belgium) and San Diego (California, USA). We provide comprehensive process development services, current good manufacturing practice (CGMP) manufacturing, and drug-product fill–finish services of small-molecule and biologic-active pharmaceutical ingredients (APIs) and intermediates.
Fill out the form below to read this capabilities review now.
Avid Bioservices is a full-service, dedicated contract development and manufacturing organization (CDMO) focused on development and manufacturing of biopharmaceutical products derived from mammalian cell culture. Avid provides process development and current good manufacturing process (CGMP) clinical and commercial product manufacturing and offers expertise supporting analytical development, qualification, and validation activities. Additional service offerings include cell line optimization, cell culture and feed optimization, product characterization, and stability testing. Avid has 26 years of experience producing a comprehensive range of proteins, including monoclonal antibodies and recombinant proteins in batch, fed-batch, and perfusion modes as well as bispecific monoclonal antibodies, Fc-fusion proteins, and biosimilars.
Fill out the form below to read this capabilities review now.
Autologous T-cell therapies no longer are limited to small, single-site trials. National and international demand is growing, bringing with it the demand for rapid scaling of manufacturing. But there is little margin for error, because any variables in an end-product could result in a potentially hazardous change to a therapy’s potency.
Cryopreservation is a critical tool for quality control and transport of cells. Liquid nitrogen (LN
2
)–based controlled-rate freezers have long been used to freeze clinical-grade T cells. Cells are frozen in either cryogenic vials or bags, using LN
2
to cool them at –1 °C/min to a desired temperature. Cryopreserved cells then can be transported
or stored long term. Unfortunately, use of liquid nitrogen creates a safety risk of oxygen depletion in the room where it is used. Therefore, appropriate infrastructure and safety
systems must be installed within a cleanroom environment. This can be expensive and cumbersome.
Fill out the form below to read the complete white paper...
Oncolytic viruses constitute a new promising therapeutic approach for treating cancer. These viruses selectively replicate in tumor cells and effectively kill those cells without harming normal cells. Selectively engineered, the viruses do not only destruct target tumor cells, but they also stimulate the host’s antitumor immune response.
Adenoviruses are extensively characterized, well-studied
viral vectors that infect both dividing and nondividing cells without the risk of integration into the host genome. Generally causing a mild nature of disease, adenoviruses are considered to be safe delivery vectors for gene therapy applications. Recently, oncolytic adenoviruses have been applied successfully as cancer immunotherapies or tumor vaccines. As one of the most studied vectors for experimental and clinical use, adenovirus serotype 5 (AdV5) is a suitable system for development of a process for oncolytic adenovirus production.
Fill out the form below to read the complete white paper now.
The field of cell and gene therapy is transforming the way patients who are diagnosed with cancers or genetic diseases can be treated. Today, the cost of producing these therapies still represents a major hurdle on the way to the commercialization. New technologies are needed that enable robust and cost-efficient manufacturing and yield replicable high-quality medicines. Although the therapeutic opportunities for patients are exciting, the stakes for patients and drug developers are high.
We want to be your partner, add value to your therapy development process, and drive pioneering therapies to the market. We invest in enabling technologies and build expertise to support the development and commercialization of new innovative therapies. Our scientists and engineers bring decade-long development experience across a broad spectrum of cell types and technologies. That expertise builds the backbone of an extensive service offering, providing you with tailored process and analytical development, manufacturing...
Rentschler Biopharma SE, a leading contract development and manufacturing organization (CDMO), has been at the forefront of biologic development and manufacturing for over 40 years. Our value chain encompasses the whole process: from gene to vial and from concept to market. As a full-service provider, Rentschler Biopharma offers its clients one primary contact, with only one contract signed for all phases of a development cycle. As a provider of high-end solutions for our partners, we help clients transition a project from genetic engineering all the way through fill and finish. Projects are supported by analytical method development and validation, formulation development, and the elaboration of optimal global regulatory approval strategies from the very beginning, through clinical studies up to market approval.
Fill out the form below to read the complete capabilities review now.
Wacker Biotech is “The Microbial CMO” — the partner of choice for process development and contract manufacturing of biopharmaceuticals (proteins, vaccines, and live microbial products) using microbial hosts. WACKER’s integrated service portfolio covers molecular biology, process/analytical development, and good manufacturing practice (GMP) manufacturing of biologics for clinical and commercial supply.
WACKER operates three state-of-the-art GMP facilities located in Europe (Germany and The Netherlands). Manufacturing lines with two 300-L and two 1,500-L stainless steel fermentation vessels, single-use fermentation lines, matching downstream processing scales and fill and finish capabilities are available to suit all possible customers’ needs (BSL 1 and 2).
WACKER holds biomanufacturing certificates from the relevant authorities for all sites and follows the ICH Q7A guidelines for GMP-compliant production of active pharmaceutical ingredients (APIs) and drug products (DPs). All three GMP production facilitie...
The development of a new, innovative drug is a highly complex and cost-intensive technical task. It normally takes more than 10 years from initial idea to first approval of a drug. As the statutory requirements for patient safety have become ever more stringent in recent years, development expenditure has increased significantly. High investment costs needed to develop some drug products are estimated to be 1–1.6 billion US dollars, which can be amortized only after market launch (
1
). The race against time for patients and valuable new active agents begins long before a new drug can be
used to save lives.
Given the time pressure to achieve rapid market launch of a drug, innovative solutions are called for to reduce project lead times and to meet ever-increasing quality requirements. The scale-up process for producing active pharmaceutical ingredients (APIs) requires a wide range of outstanding engineering skills, all of which ZETA can
provide optimally by means of system-controlled knowledge transfer. Z...
Pharmaceutical manufacturing is an industry laden with many challenges and risks because of the life-changing nature of its products — drugs that can affect millions of human lives. Manufacturers perform immensely complex research and testing daily while facing economic challenges and regulatory requirements. This article covers the benefits of surface plasmon resonance (SPR) in the drug development pipeline and describes a recent validation guide specifically for the implementation of SPR technology in regulated workflows.
Fill out the form below to read the complete white paper now.
Host cell proteins (HCP) constitute a major group of impurities for biologic drugs produced using cell culture technology. Even at nanogram per milligram concentrations of HCP to drug substance (DS), HCPs can elicit undesired immune response, interfere with drug safety and efficacy, or impact DS stability.
A broadly-reactive HCP ELISA should be used during the purification processes to ensure removal of HCPs and to demonstrate process consistency and final DS purity. Regulatory authorities are requesting biopharmaceutical companies employ orthogonal methods to demonstrate antibody coverage to individual HCPs and provide a comprehensive assay qualification package to ensure the HCP ELISA used by a sponsor is fit for this purpose.
Antibody Affinity Extraction™ (AAE™), a novel method developed by Cygnus Technologies, is used to determine antibody coverage and reactivity to those HCPs that co-purify with DS. AAE is more predictive of the anti-HCP antibody performance in the HCP ELISA and facilitates identific...
Sponsored Content
Single-Cell Dispensing for Cell Line Development and Single-Cell Genomics: Most Trusted Technology for Assuring Monoclonality by the Pharmaceutical IndustrySingle-Cell Dispensing for Cell Line Development and Single-Cell Genomics: Most Trusted Technology for Assuring Monoclonality by the Pharmaceutical Industry
Clonal cell line development is a crucial step in a number of applications, including generating biopharmaceuticals (e.g., monoclonal antibodies, MAbs). Current workflows in cell line development have major drawbacks such as missing proof of clonality, inefficient single-cell isolation, and reduced cell viability. The single-cell printer™ technology offers documented assurance of clonality and provides efficient and fast single-cell seeding combined with excellent cell viability and zero risk of cross-contamination. All cytena products support SLAS/SBS format 96-well and 384-well plates. Different typical cell lines used in cell line development such as CHO-K1, HEK 293, L929 can be processed.
Fill out the form below to read the complete capabilities review now.