Niche Disease: CAPS
by Cheryl Scott
Cryopyrin-associated periodic syndromes (CAPS) are rare, inherited, autoinflammatory diseases with similar genetics and overlapping symptoms. The group includes three subtypes: familial cold autoinflammatory syndrome (FCAS); Muckle-Wells syndrome (MWS); and neonatal-onset multisystem inflammatory disease (NOMID), also called chronic infantile neurologic cutaneous articular (CINCA) syndrome. The cryopyrin protein is critical to production of the inflammatory cytokine interleukin-1β (IL-1β), which triggers inflammatory response when it binds to inflammatory cells. Genetic alterations in cryopyrin cause IL-1β overproduction, resulting in an inflammatory response and the symptoms of CAPS.
FCAS symptoms can develop when patients are exposed to even a mild degree of cold temperature. A systemic inflammatory response usually ensues within a few hours with rash, fever/chills, arthralgia, conjunctivitis, and fatigue. Most flares last <24 hours. MWS has similar inflammatory sympt...
Fotoproduktion bei Sartorius, Göttingen vom 04.12.-10.12.11
Increased commercial use of single-use systems (SUS) for large-scale biopharmaceutical production creates the need for consensus on industry best practices and standards for materials in SUS components. End users and suppliers are beginning to develop a shared vision of industry needs in such areas (
1
,
2
). For example, highly visible efforts to harmonize extractables testing include contributions from groups such as the BioPhorum Operations Group (BPOG), Bio-Process Systems Alliance (BPSA), Parenteral Drug Association (PDA), ASTM, and ISPE.
In addition to extractables testing, similar collaborative efforts focus on particulates, integrity testing, interchangeability of materials/components, and supplier qualification requirements. We build on this vein of current industry work, discussions, and recent publications by proposing development of a new standard assay for assessing the suitability of specific plastics in cell-growth applications fo...
GRAPHIC STOCK (WWW.GRAPHICSTOCK.COM
The biopharmaceutical manufacturing industry continues to expand rapidly, with growth in revenue for both suppliers and drug innovators averaging about 15% per year. Understandably, much of the industry’s recent focus has been on the pace of technological improvements that have boosted productivity and performance. But another issue is becoming just as critical: The flow of skilled staff able to keep up with the industry’s growth isn’t keeping pace. Results from our 2015 annual industry study suggest that the hiring market remains tight, with hiring difficulties increasing in some key areas (
1
).
My group surveyed 237 qualified individuals at biopharmaceutical manufacturers and contract manufacturing organizations (CMOs) across 28 countries (see “Study Methodology” box). As part of our study, we delved into the state of hiring, employment growth, and training in biopharmaceutical manufacturing.
Results reflect a trend over the past several years: With more products con...
GRAPHIC STOCK (WWW.GRAPHICSTOCK.COM)
Qualified scale-down models of large-scale cell culture processes are essential to conducting studies for applications such as investigating manufacturing deviations, enhancing process understanding, and improving process robustness. For example, scale-down models can be used for raw material investigations as well as evaluation and qualification of new good manufacturing practice (GMP) cell banks for manufacturing implementation. Process characterization studies are performed also with qualified scale-down models to improve process consistency (
1
,
2
). Often it is impractical to conduct investigational studies at large scale; therefore, results gathered from a qualified scale-down model are valuable as predictors of at-scale performance.
One important consideration in developing a small-scale model is to identify which parameters depend on or are independent of scale. Scale-dependent variables are adjusted to accommodate for scale differences such as vessel geometr...
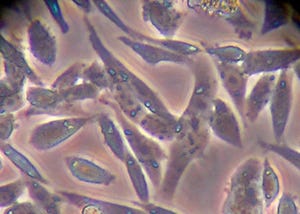
Subconfluent monolayer of Chinese hamster ovary (CHO) cells under high magnification using phase-contrast microscopy (40× objective); typical cellular morphology of cells grown for five days in cell culture medium at 37 °C. PHOTOMICROGRAPH COURTESY OF PATRICK J. CUMMINGS AND KRISTINA M. OBOM AT JOHNS HOPKINS UNIVERSITY (AMERICAN SOCIETY FOR MICROBIOLOGY MICROBE LIBRARY) (WWW.MICROBELIBRARY.ORG)
The first recombinant protein licensed for use by the United States Food and Drug Administration (US FDA) was human insulin in 1982 (
1
). That approval was followed in 1987 by the development of tissue plasminogen activator (tPA), the first complex glycosylated protein generated in mammalian cells to be licensed for therapeutic use. Since then, this area of biology has rapidly expanded in clinics: The FDA approved an average of 15 new biological entities every year between 2006 and 2011 (
2
). Although many systems have been developed to generate biologics over the past 25 years, Chinese hamster ovary (CHO) cells ...
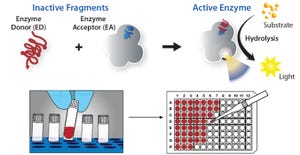
Figure 1: DiscoveRx’s proprietary PathHunter enzyme fragment complementation (EFC) technology consists of the β-galactosidase (β-gal) enzyme split into two inactive components, the enzyme donor peptide (ED) and an enzyme acceptor (EA). When brought together in close proximity, ED complements with EA to form active β-gal. The active enzyme then catalyzes the substrate to generate chemiluminescent light, providing a highly amplified signal to make for a high-sensitivity assay.
With the drug industry’s expanding emphasis on biologics, the need for robust cell-based assays has grown at all stages of development. Requirements for efficacy, quality, and potency testing often demand a complex set of bioassays and/or cell-based assays for new therapeutics or biosimilars. Developers of the latter have found this need for cell-based assays to be particularly challenging.
Commercially available, ready-to-use cell-based assays provide a robust functional response from specific therapeutic targets. They can significan...
with Dr. Andreas Anton and Dr. Sebastian Schuck
Poorly soluble substances form aggregated inclusion bodies (IBs) in microbial cells containing incorrectly and/or incompletely folded target proteins. Wacker Biotech, a full-service contract manufacturer of biopharmaceuticals based on microbial systems, has introduced FOLDTEC refolding technology for bioengineered therapeutic proteins. The proprietary platform uses specifically developed and optimized bacterial strains and a patented, antibiotic-free expression system.
In a BPI webinar on 9 November 2015, Wacker’s director of bioprocess development (Andreas Anton) and head of business development (Sebastian Schuck) demonstrated the potential of this technology with a case study of recombinant expression and in vitro refolding of thrombin, a difficult-to-manufacture protease used to control bleeding.
The Presentations
Schuck introduced FOLDTEC
Escherichia coli
folding technology for refolding IBs. He described it as “based on three pillars”: proprietary ant...
Biopharmaceuticals are the fastest growing sector of the pharmaceutical industry, making up about 20% of the market, with annual growth rates of about 8% (double that of more traditional pharmaceutical sectors). To increase capacity and uphold stringent quality and regulatory demands, manufacturers often reassess their operational and technology strategies while focusing on rising manufacturing costs and the pressure of delivering cost-effective new drug products.
Bioproduction can range from small batches to low-cost, high-volume campaigns. Few manufacturers have the required in-house capacity for such a range, so they engage smaller, more agile contract manufacturing organizations (CMOs) and/or adapt multipurpose facilities with modular process concepts and single-use equipment to minimize cross contamination. And advanced process technologies enable standardized functional process modules, which help manufacturers simplify, verify, and reuse designs. Such flexibility allows functionalities to be added ...