Heading for a CHO Revolution: The Need for Cell Line Engineering to Improve Manufacturing Cell LinesHeading for a CHO Revolution: The Need for Cell Line Engineering to Improve Manufacturing Cell Lines
January 13, 2016
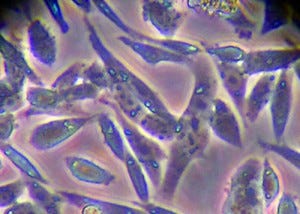
Subconfluent monolayer of Chinese hamster ovary (CHO) cells under high magnification using phase-contrast microscopy (40× objective); typical cellular morphology of cells grown for five days in cell culture medium at 37 °C. PHOTOMICROGRAPH COURTESY OF PATRICK J. CUMMINGS AND KRISTINA M. OBOM AT JOHNS HOPKINS UNIVERSITY (AMERICAN SOCIETY FOR MICROBIOLOGY MICROBE LIBRARY) (WWW.MICROBELIBRARY.ORG)
The first recombinant protein licensed for use by the United States Food and Drug Administration (US FDA) was human insulin in 1982 (1). That approval was followed in 1987 by the development of tissue plasminogen activator (tPA), the first complex glycosylated protein generated in mammalian cells to be licensed for therapeutic use. Since then, this area of biology has rapidly expanded in clinics: The FDA approved an average of 15 new biological entities every year between 2006 and 2011 (2). Although many systems have been developed to generate biologics over the past 25 years, Chinese hamster ovary (CHO) cells have become the predominant expression system for manufacturing complex glycoproteins.
Although protein yield achieved with CHO cells has increased about 100-fold over the years (3), advances in cell line technology are still limited by the biology of these cells. Fundamental improvements to CHO cell lines are therefore needed to increase the potential of host systems.
To that end, gene editing can offer a significant step change in biopharmaceutical manufacturing. As the pharmaceutical industry moves toward an ever more fragmented market (driven by the movement toward personalized medicine), smaller manufacturing batches must become economically viable. Equally, as therapeutics based on novel protein architecture are developed, modifications of such systems are needed to maximize benefits derived from exciting new therapies.
The CHO Advantage
Many expression systems have generated biologics over the past 25 years. Prokaryotic systems are still primarily used for simple proteins that do not require complex glycosaccharides for their efficacy or stability. Biologics that need complex posttranslational modifications require mammalian hosts such as CHO cells, which are the predominant expression system. The safety implications of using animal-derived products to culture production cells has led manufacturers to prefer cells that are grown in serum-free conditions in chemically defined media. Concomitantly, the manufacturing advantage conferred by using cells that can grow at high density in suspension culture has led to the industry’s preference for such cells.
The natural characteristics of CHO cells have contributed to their popularity. They are somewhat easily adaptable to suspension growth in animal-component–free, chemically defined media, and they express and secrete recombinant proteins (including antibodies) at relatively high levels and provide a “human-like” glycosylation profile. This latter characteristic prevents activation of patient immune responses that can lead to unwanted side effects or rapid clearance of a drug. By comparison, murine cells can induce immunogenic effects through their incorporation of an alpha-galactose residue (4). Once stably transfected with a vector-expressing recombinant protein, CHO cells generate a heterogeneous population of cells. Some CHO cells express high levels of a protein of interest through a combination of integration sites and the ability of these cells to amplify regions of genomic DNA.
Extensive optimization of culture media and feeding strategies is such that expression levels have increased about 100-fold over initial capabilities. Some alterations to CHO cells themselves have decreased the time required to identify the highest-expressing clones, specifically through metabolic selection systems. Such modifications have focused on selecting cells that have integrated the expression cassette into sites that enable high expression of recombinant product. Initially, this was achieved through the use of the methotrexate/dihydrofolate reductase (MTX/DHFR) system and then the use of the methionine sulphoximine/glutamine synthetase (MSX/GS) system. The GS selection system is more stringent than DHFR, leading to reduced timelines for identifying high-expressing clones (5).
However, such advances in cell line technology are still limited by the natural biology of CHO cells. A selection system can identify only those clones that naturally express higher levels of protein. And media optimization can ensure only that those clones selected are in the best possible health to produce maximal amounts of protein. To increase the potential of a host system, something more fundamental must be improved.
The Need for Further Advancement
Whether further advancement is necessary is a valid question. After all, expression titers are higher than ever, and the time needed to isolate production clones is now measured in weeks instead of months or years. However, treatment courses of biotherapeutics are more than 20 times more expensive than they are for small-molecule therapeutics (6). The CHO expression system is being pushed to its limits as manufacturers strive to make smaller batches economically viable. Such efforts are needed to keep pace with the development of ever more personalized approaches to medicine. Patients are stratified into ever smaller groups as mechanisms of disease progression are linked to individual genotypes, which leads to improved therapeutic outcomes for different groups of patients. The same economic argument can be applied to orphan diseases, with clear benefits to sufferers of such relatively rare conditions.
Monoclonal antibody (MAb) production in CHO cells has been the focus of many biological processes. MAb expression at multiple grams per liter of culture fluid is becoming the industry norm. However, the bioindustry is increasingly shifting toward novel classes of antibody-based therapeutics. Even with the increased potency afforded by new technologies, expression levels often are too low to economically produce an active protein.
Existing therapies could benefit from improvements in manufacturing platforms. Regulatory acceptance of biosimilars and their large potential markets following patent expirations have led to a dramatic increase in the number of companies entering this field. The current estimated cost of some biosimilars is about half of that for the related originator products, so potential savings in manufacturing costs are attractive. When producing a biosimilar, similarity of posttranslational modifications to the originator product is one of the most challenging aspects to address. Currently that is achieved through process modification, but control over aspects such as glycosylation would be a beneficial feature of a production platform.
Although some companies have stated that they will keep manufacturing of complex products in house (7), the bioindustry is trending toward outsourcing manufacturing (8). That has led to the emergence of many contract manufacturing organizations (CMOs) with expertise in biologics manufacturing. CMOs require efficient use of flexible facilities to maximize the use of existing capacity.
In new facilities, multiple smaller bioreactors are now the norm to meet market need. By contrast, large legacy plants contain tens of thousands of liters of capacity. The “downsizing” trend has also been enabled by increased adoption of single-use bioreactors, which are (in some cases) taking the place of large stainless steel bioreactors. The same is true for small biotechnology companies wishing to keep manufacturing in house.
The natural extension is a trend toward decreased production scales across the industry (9). If cell lines can generate high-yielding clones that produce a required amount of protein at decreased scale, this process would fit with a movement toward smaller scale production. Similarly, decreasing run times of bioreactor batches would increase process flexibility and capacity of existing plants.
Clearly, improved production systems that increase manufacturing efficiency or control over posttranslational modifications are attractive to a number of biomanufacturers with different priorities. To achieve that goal, existing options are to alter the CHO cell platform to allow improved expression or to change systems altogether for one that has an improved basal capacity.
A number of different systems are available, each with its own characteristics. For example, plant cells scale easily and produce high titers (as do insect cells), but a number of concerns limit uptake of novel systems. From a practical perspective, those systems are competing with an extensive history of production of safe therapeutics. So drug makers still have concerns over nonmammalian glycosylation moieties.
Biomanufacturers have a lot of experience in processes that use CHO cells. CHO systems are incredibly well characterized, with known safety profiles and well-established processes to ensure product quality and batch consistency. In addition, more scientists are trained in handling CHO cells than any other expression system. So changing the system would require significant time and resource investment. Thus, there is a strong case for the industry not to move away from CHO cells, but rather fundamentally improve existing systems.
Engineering CHO Cell Lines
One CHO-line improvement strategy that is gaining traction is engineering that line — all while maintaining the characteristics that have led to it becoming the system of choice. My company suggests that the bioindustry has a great opportunity to revolutionize current CHO platforms by altering the genome. Company researchers have begun an extensive program to achieve this objective, and their avenue of research could have significant implications for future bioprocessing.
Given the correct targets, genome engineering to improve CHO-cell expression systems has almost limitless potential. The glutamine synthetase (GS) system is the current industry-standard selection system. To encourage industry innovation in this field, we are facilitating access to CHO cells by changing current licensing paradigms. These cells will then be targeted through further engineering to remove the need for a completely alternative system.
A number of different approaches can be taken to improve the GS system. Potential modifications range from whole-gene knockouts or knock-ins to individual point mutations on target genes (which affect activity of target enzymes or substrates). Multiplexing many different engineering events within one line will synergistically affect target phenotypes.
For example, you could reasonably anticipate that manipulating both transcription and translation would have a multiplicative effect on specific productivity. Alternatively, regulation of product quality could be fine-tuned by controlling transit through the endoplasmic reticulum (ER) as well as careful modification of chaperone proteins. It will even be possible to introduce entire new molecular pathways. Trait stacking these improvements onto an existing cutting edge line can encourage industrywide uptake of these cells. Through licensing and performance, these modified cells can become a platform technology that leads to further improvements, innovation, and optimization within different aspects of process development. And innovation through engineering such high-performance cells for specific applications can lead to a panel of different cell lines, each optimized to overcome specifically designated challenges.
Although the potential of cell line engineering is nearly endless, the practicalities are currently limited. Not only do the best phenotypes need to be identified, but the correct targets then must be established. That has recently been enabled by the availability of omics data, which have improved understanding of CHO biology (10–12). But the industry still needs greater understanding of the implications of new omics information. Mathematical modeling is rapidly evolving, and it promises a positive effect on the ability of researchers to predict the impact of particular modifications. However, investment in bioinformatics is needed to keep pace with the ambitions of the industry.
Because CHO cells can adapt to different pressures, many different proteins in one cell must be modified before a strong effect can be observed. For example, the complex nature of the metabolic network means that the system has a lot of redundancies, so modifications of many different aspects might be needed to achieve a desired result. The bioindustry is now at the point where increased understanding of CHO biology through interpretation of available omics information allows us to identify best targets. Once correct targets and modifications are identified, the industry’s ability to introduce them to the cells will not become the main concern.
The discovery of the CRISPR/Cas9 system (13) is driving a surge in interest in engineering technologies. This system can improve the stacking of engineered modifications, and it has already been used to multiplex engineering events (14). Because of its relatively recent discovery, the CRISPR/Cas9 system is still evolving rapidly. Scientists are improving the specificity and efficiency of this exciting new technology, which is making genome engineering more accessible than ever before. The potential of genome engineering has driven the sequencing of the CHO genome, which has allowed scientists to design efficient targeting vectors.
I must stress that at this stage, the intellectual property surrounding CRISPR engineering has yet to be resolved. So although the technology currently has much potential, its use is restricted to research and development. However, other technologies are available to realize the potential of cell-line engineering. For example, induction of homologous recombination through the use of recombinant adeno associated virus (rAAV) is an established technology. But the nature of rAAV directs that only one allele can be targeted at a time, which makes the introduction of multiple events a time-consuming process. Zinc finger nucleases (ZFNs) work on principles similar to those of the CRISPR/Cas9 system, but they have lower efficiencies than CRISPR (15) as well as specificity concerns (16). Aside from this, access to ZFNs can be cost prohibitive to the majority of companies for use in biomanufacturing. Finally, directed evolution or random mutagenesis can be used (17), but care must be taken to avoid unwanted mutations that can lead to unintended consequences.
Antibody engineering can greatly influence biotherapeutics manufacturing. It is becoming apparent that substitutions of only a few amino acids in an antibody sequence can affect not only potency, but also expression and aggregation. Similarly, modifications of signal peptides can significantly increase levels of secreted protein. Although a full discussion of the potential of this area is beyond the scope of this article, combining optimization of the cell line with codeveloped expression vectors could have a significant impact on the field in the future.
Goals in Mind
Cell line engineering has great potential for improving existing CHO cell expression systems in a number of ways beyond what is currently available. This is not a simple enterprise, and it’s not without its risks. But the reward could be the ability to cost-effectively produce biotherapeutics. In turn, that would make not only existing therapies more affordable, but it would allow small production runs to work toward the realization of personalized medicine.
References
1 A History of Firsts. Genentech: South SanFrancisco, CA; www.gene.com/media/ company-information/chronology.
2 Lai T, Yang Y, Ng S. Advances in Mammalian Cell Line Development Technologies for Recombinant Protein Production: Pharmaceuticals. Multidisciplinary Digital Publishing Institute 6(5) 2013: 579–603.
3 Hacker DL, Nallet S, Wurm FM. Recombinant Protein Production Yields from Mammalian Cells: Past, Present, and Future. BioPharm Int. 21, 2008: S6–S14.
4 van Beers MMC, Bardor M. Minimizing Immunogenicity of Biopharmaceuticals By Controlling Critical Quality Attributes of Proteins. Biotech. J. 7(12) 2012: 1473–1484.
5 Fan L, et al. Improving the Efficiency of CHO Cell Line Generation Using Glutamine Synthetase Gene Knockout Cells. Biotechnol. Bioeng. 109(4) 2011: 1007–1015.
6 Opportunities for Biosimilar Development. Generics and Biosimilars Initiative: Mol, Belgium; www.gabionline.net/Biosimilars/Research/Opportunities-for-biosimilardevelopment.
7 Stanton D. Novartis to Keep Complex Biologics Manufacturing In-House. BioPharma Reporter, 21 November 2014.
8 Langer E. Biopharmaceutical Outsourcing Continues to Expand. Pharma. Outsourcing, 28 January 2014.
9 Jagschies G. Where is Biopharmaceutical Manufacturing Heading? BioPharm Int. 21(10) 2008.
10 Brinkrolf K, et al. Chinese Hamster Genome Sequenced from Sorted Chromosomes. Nature Biotech. 31(8) 2013: 694–695.
11 Lewis NE, et al. Genomic Landscapes of Chinese Hamster Ovary Cell Lines As Revealed By the Cricetulus griseus Draft Genome. Nature Biotech. 31(8) 2013: 759–765.
12 Kildegaard HF, et al. The Emerging CHO Systems Biology Era: Harnessing the ‘Omics Revolution for Biotechnology. Current Opinion in Biotechnology 24(6) 2013: 1102–1107.
13 Mali P, Esvelt KM, Church GM. Cas9 As a Versatile Tool for Engineering Biology. Nature Methods 10(10) 2013: 957–963.
14 Grav LM, et al. One-Step Generation of Triple Knockout CHO Cell Lines Using CRISPR/Cas9 and Fluorescent Enrichment. Biotechnology J. 10(9) 2015: 1446–1456.
15 Perkel J. Genome Editing with CRISPRs, TALENs, and ZFNs. 27 August 2013; www.biocompare.com/EditorialArticles/144186-Genome-Editing-withCRISPRs-TALENs-and-ZFNs.
16 Pattanayak V, et al. Revealing OffTarget Cleavage Specificities of Zinc-Finger Nucleases By In Vitro Selection. Nature Methods 8, 2011: 765–770.
17 Meuris L, et al. GlycoDelete Engineering of Mammalian Cells Simplifies N-glycosylation of Recombinant Proteins. Nature Biotech. 32(5) 2014: 485–489.
Jamie Freeman, PhD, is bioproduction product manager at Horizon Discovery, 7100 Cambridge Research Park, Waterbeach, Cambridge CB25 9TL, United Kingdom; [email protected].
You May Also Like






