In my short time as BPI’s resident novice, I have been impressed with this industry’s interdisciplinarity and collaborative spirit. Executives tell me that biopharmaceutical companies have not always been so multifaceted in their expertise or so willing to forge partnerships across organizations and over the industry–academia divide. I am lucky to have started working with you when I did late in 2019.
That is not to regard the biopharmaceutical industry with rose-tinted glasses. Many elements of biomanufacturing remain closely guarded secrets, and competition across businesses remains lively. But even before the COVID-19 pandemic, I noticed during conferences and interviews how much life-science professionals want to share what they know, learn what they don’t, and invite scientists from other organizations and fields to join the conversation.
For that, I thank you. Rapid development and testing of SARS-CoV-2 vaccines and antivirals has required a shared sense of purpose, and the industry will need such i...
Innovations in bioproduction of therapeutics over the past 20 years have led to impressive improvements in product yield, process controls, and manufacturing safety. Industry 4.0 concepts have been embraced across the bioprocess industry and are leading to better bioprocess control through process automation, “big data” and data analysis, process simulations, the industrial internet of things (IIoT), cybersecurity, the cloud, blockchain/serialization, and additive manufacturing. Such advances help to ensure that a process results in the same outcome every time. As Sean Werner (president of Sexton Biotechnologies) commented in a video interview from last summer, “We can’t control the inherent variability in biology, but we do understand a few of the components of the process that we can control” (
1
).
In the highly regulated field of biotherapeutics manufacturing, even process changes that lead to improvements can require process revalidation and therefore be perceived as generating risk. However, the big...
The life-sciences industry has been working hard to meet the deadline for compliance with the European Union’s Medical Device Regulation (EU MDR, 2017/745) (
1
). Doing so has been a challenging journey for many companies. Therefore, the full-year postponement of the final application date has been a welcome development, particularly in view of the new and extraordinary challenges stemming from the COVID-19 global health crisis. The extension has instigated other important changes, so it is critical that life-science businesses familiarize themselves with their implications.
Key Takeaways for the Medical-Device Industry
In a 17 April 2020 amendment to the original regulation (
2
), the European Parliament approved by an overwhelming margin the new deadline of 26 May 2021. That delay applies to the EU MDR only, with no corresponding changes to the In Vitro Diagnostic Regulation (IVDR, 2017/746) (
3
). Likewise, regulatory requirements remain the same for medical-device manufacturers, notified bodies, autho...
Process validation is a key part of the development and manufacture of all approved drug products, but its completion can be a daunting task. At a two-day CASSS CMC Strategy Forum held in July 2016 in Gaithersburg, MD, speakers and attendees addressed the many technical, practical, and regulatory facets of drug product process validation and comparability. In part 1 of this report, we summarize the key discussion points of the first day, which focused on analytics and comparability.
Session One: Analytical Tools for Drug Products
Container–Closure Integrity Testing and Control:
Michael Koby (Pfizer) started his presentation with a summary of the BioPhorum Operations Group (BPOG) publication on container–closure integrity (CCI) control and its comparison with integrity testing during routine manufacturing (
1
). The white paper describes the evolution of the CCI testing (CCIT) landscape, including a push toward 100% in-line CCIT during manufacturing.
Koby mentioned that the latest revision of USP Chapter ...
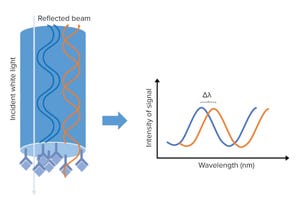
Figure 1: A biolayer interferometry (BLI) biosensor and its optical properties; left side shows the tip of the BLI biosensor, with a molecular binding event on the tip. White light is sent to the sensor tip, where it is reflected on an internal reference layer and on the molecular surface. Protein interaction causes a wavelength shift (∆λ) between the reflected beams. The ∆λ will be the output of the assay and reflect the change in mass on the sensor surface, allowing detection, quantification, and kinetic evaluation of protein interactions.
Biopharmaceuticals are the largest group of drugs under development (
1
), and the demand for new and safe drug products is high. The most common bacterial and mammalian cell lines for production are
Escherichia coli
, Chinese hamster ovary (CHO) cells, and yeast.
During a production bioprocess, a cell line expresses not only the molecule of interest, but also host-cell proteins (HCPs). They are considered to be impurities in a final drug product because they can aff...
Cell culture medium is critical to cell growth, metabolism, and protein expression. It provides for optimum pH, osmolality, and nutrients in an environment that is essential for cell survival, growth, and expression of proteins and/or metabolites and drug-substance modalities of interest (
1
). A complete medium typically contains basic nutrients such as carbohydrates, amino acids, lipids, salts, vitamins, trace metals, growth factors/hormones (e.g., insulin), antishear factors, and other chemicals that facilitate cell growth and protein expression and may stabilize recombinant protein products secreted from cells. Many formulations currently used by biopharmaceutical companies are based on modifications to classical media compositions such as Dulbecco’s Modified Eagle’s Medium (DMEM) and Ham’s F12 media.
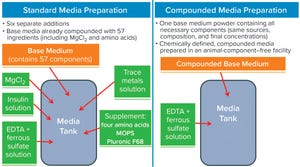
Figure 1: Standard medium preparation (left) and process using compounded medium (right); most (
57
) medium components are in the base media powder, with additional components added in house to complete...
Cell-based advanced therapies are changing modern medicine dramatically. Immunotherapies such as chimeric antigen receptor (CAR) T-cell therapies are treating different forms of cancer. Gene therapies are reversing the course of inherited diseases, and tissue-engineered medical products are restoring, maintaining, and replacing damaged organs (
1–4
). The development of new advanced therapies is booming. As of January 2020, the US Food and Drug Administration (FDA) has reported more than 900 investigational new drug (IND) applications for cell and gene therapy products.
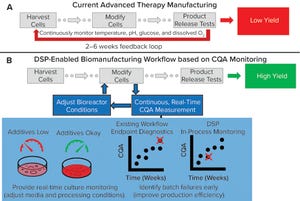
Figure 1: Comparing a representative state-of-the-art manufacturing workflow and one enabled by the dynamic sampling platform (DSP); (a) for cell therapy production, cells are harvested from a patient (autologous) or donor (allogeneic) before undergoing numerous steps, including those that “modify” cells to become medically potent. After a long production process, release assays are performed to determine whether a drug product can be deli...
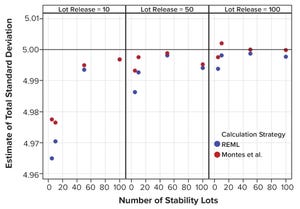
Figure 1: Estimates of total standard deviation from simulated results; REML is restricted maximum likelihood, and Montes et al. is in Reference
1
.
A reasonable estimate of long-term variation for a biopharmaceutical product critical quality attribute (CQA) can be challenging to justify, especially in the early stages of a product’s lifecycle when only limited data are available. However, if the combination of product and analytical method reasonably can be matched with historical data, prior knowledge can provide an estimate of a value. This variation estimate could be used to assist in risk assessments related to continued process verification (CPV) activities, including control charting and estimating manufacturing capability. The study below uses simulated data to suggest a calculation strategy for analyzing prior-knowledge data, with estimates of variance components.
Sources of Variation
When estimating the total variance for a CQA, analysts should include stability data (at the recommended storage...
SNAP columns for fast-protein and high-performance liquid chromatographies (FPLC, HPLC), from Astrea Bioseparations
On 10 November 2020, BPI presented an “Ask the Expert” webinar with Dan Yukon (head of North American and global SNAP product sales at Astrea Bioseparations) on considerations for selecting analytical fast-protein liquid chromatography (FPLC) columns. With many options on the market, deciding which type and brand to use can be difficult. To help take out the guesswork, Yukon addressed a number of topics, including pressure and volume considerations; column configuration; materials of construction; frit type, design porosity, and mounting; connection types; adjustability; construction accuracy; packing tube design; and column storage.
Yukon’s Presentation
Speaking from a mechanical engineering background, Yukon explored general considerations for column selection assuming
Volume
— of both resin and sample — is the core starting point, Yukon stated. Different resins capture different sample v...
Ben Josey, PhD (field application scientist at Corning Life Sciences), joined BPI on 3 December 2020 to deliver an “Ask the Expert” presentation about using optical-sensor–guided centrifugation for cell therapy development. Cell-separation techniques fall into four basic categories: filtration, centrifugation, affinity purification, and emerging methods such as microfluidics and acoustofluidics. Selecting the technology best suited to an application requires careful balancing of method precision and process efficiency, the latter of which includes factors such as batch size, time, labor, and cost. Josey described how the Corning X-Series cell-separation platform helps to strike such a balance by adding intelligent sensor control to centrifugation.
Josey’s Presentation
Josey noted that centrifugation is a conventional approach to processing blood products for cell therapy production. Centrifugation accelerates stratification of different cell types into distinct density layers. Traditionally, scientists ha...
On 10 December 2020, BPI presented an “Ask the Expert” webinar with Jason Sterling, PhD (principal scientist and project director in analytical and formulation resources), and John Rockwell (group leader) of Catalent Pharma Solutions. Biophysical characterization is critical to understanding the makeup and behaviors of biologic therapies and vaccines both early in development and throughout scale-up for manufacturing. As biologics become more complex in structure and as scientists improve their understanding of the effects of structure on stability, efficacy, and safety, new and improved analytical methods for characterizing biologic products must be developed.
The experts discussed challenges in biophysical characterization and presented solutions for overcoming them. Sterling reported on three common techniques that are used to identify, characterize, and quantitate sialic acids; Rockwell highlighted a method for evaluating extractables and leachables (E&L) to measure residual-free toxins during antibod...
Ashleigh Wake began her 15 October 2020 “Ask the Expert” presentation by pointing out that peptide products are manufactured in a “regulatory vacuum.” Peptide-product developers must be strategic in designing characterization and quality control (QC) programs. Wake reviewed available methods and explored key considerations for developing phase-appropriate analytical controls.
Wake’s Presentation
Because peptides overlap small- and large-molecule drugs in size, regulatory expectations differ by product size and clinical indication. Thus, analytical programs should be designed around critical quality attributes (CQAs) that influence peptide-product safety and efficacy. Deep understanding of a peptide’s structure, physicochemical properties, impurity profile, and potential for interaction with excipients can help to delineate analytical requirements for different product-development stages.
Structural Analysis:
Slight changes in structure can reduce a peptide’s efficacy and increase its immunogenicity. Amin...
A long-awaited (and for months withheld) evaluation of the European Union’s Orphan Drug Regulation (ODR) finally reached the interested public in August 2020 (
1
). Its publication during the public consultation of the European Commission’s “Pharmaceutical Strategy” was well planned because the latter discusses policies on access, availability, and affordability of new medicines. However, the ODR evaluation shows not only that the intentions behind the legislation have not been fulfilled, but also that its generous incentives (extension of market exclusivity and patent protection, administrative simplification of study protocols and fees as well as tax benefits) have been used to the maximum. Now every third drug approved is an orphan drug (OD) sold for “orphan” prices.
Between 2007 and 2017, 131 drugs were approved as ODs for 107 rare diseases. Twenty-two of those were approved for two or more indications and for different periods of market exclusivity — with significant return on investment over long pe...