November-December 2021
One silver lining to the cloud of meeting only virtually has been our ability to “attend” some conferences to which we’ve not made it very often in the past. For example, I enjoyed tuning into the International Society of Pharmaceutical Engineers (ISPE) 30th Annual Aseptic Processing Conference, which was held virtually this past spring. It helped me plan for our September featured report, and it provided a good general update on the current state of biopharmaceutical formulation, fill and finish. You’ll find the conclusion of a two-part article on page 50 of this issue that is adapted from a presentation given at this meeting.
Richard Friedman (deputy director of science and regulatory policy at US FDA CDER’s office of manufacturing quality) and Jean Hu Primmer (global regulatory lead at GSK Vaccines) were ISPE’s keynote speakers. Friedman highlighted risk reduction, reliability, and sustainability as drivers for modernization of sterile drug manufacturing. Primmer reported on how public–private partners...
Modern waste-to-energy plant in Oberhausen, Germany
(https://www.stock.adobe.com)
The world desires a more sustainable economy in which resources can be saved, products can be profitably used, and at the end of their useful life, component materials can be recycled into other useful products. The bioprocessing industry has made efforts to meet those goals and has learned a great deal about the role of plastic components in sustainable manufacturing. The most important lesson might be that a science-based approach is required to provide an accurate benchmark of manufacturingʼs environmental burdens. Life cycle assessment (LCA) has revealed that, in general, processes using single-use technologies (SUTs) often have smaller environmental footprints than processes based on durable systems (
1
,
2
).
A proper final assessment of either intermediate values or strategic conclusions would include consideration of uncertainty factors. Researchers can attempt to quantify those factors in a risk assessment by purs...
HTTPS://WWW.STOCK.ADOBE.COM
Biosimilars represent a significant cost-savings opportunity in the United States because they can introduce competition for some of the most expensive and widely used prescription drugs on the market: originator biologics. Several market and regulatory barriers have slowed biosimilar market entry and uptake in the United States (
1
). Some hurdles are unintentional or transitory; others are deliberately crafted by manufacturers of reference biologics to thwart competition.
Among the most significant strategic barriers to biosimilars’ entry into the US market are
patent thickets
, whereby developers of reference biologics layer weak follow-on patents upon their original claims in order to prevent competition. Although patent thickets exist in other countries, they are a particularly serious problem in the United States, where they are more prolifically applied. The strategy harms patients and payers, who stand to benefit from the savings that biosimilars can generate. But pate...
WWW.ISTOCKPHOTO.COM
The pace with which biosimilar drugs have been adopted in the United States has frustrated (and displeased) policymakers (
1
). After passage of the Biologics Price Competition and Innovation Act (BPCIA) (
2
) as part of the Affordable Care Act of 2010 (
3
), policymakers intended and expected significant reductions in expenditures for this class of biopharmaceuticals (
4
). The Federal Trade Commission (FTC) had predicted that the percentage of savings would be lower than that of the <90% reduction in costs for small-molecule generic drugs (
5
,
6
). That this has not been the case provides the policy motivation for a joint effort by the Food and Drug Administration (FDA) and the FTC to achieve that goal.
Biopharmaceuticals emerged in the latter part of the 20th century, due in large part to the recombinant DNA revolution that sparked the biotechnology industry (
7
). Although some drugs that fell within the scope of the definition of a biologic drug already were known (insulin isola...
https://www.istockphoto.com
Injectable-drug formulations for both subcutaneous and intravenous administration are designed to be consistent with the number of solutes present in human tissue. Such consistency with physiological conditions is achieved by adding an appropriate amount of salt and/or sugar to attain the desired tonicity. Care must be taken to prevent exposure of cells to either hypotonic or hypertonic formulations that could cause lysis or shrinking, respectively (
1
).
Injection of formulations that deviate from human-plasma osmolality (295 mOsm) can cause pain upon injection (
2
–
4
). Thus, a reliable way to measure osmolality is crucial to parenteral drug-product development. Osmolality is a valuable in-process parameter that can pinpoint deviations during formulation compounding and can indicate excipient coconcentration or exclusion during ultrafiltration/diafiltration (UF/DF) preparation steps (
5
). Consistent osmolality measurements of drug products provide some reassurance that thei...
https://www.istockphoto.com
In
BPI’s October 2021 issue
, part one of this review introduced the concepts of machine learning (ML) and artificial intelligence (AI), identifying some broad areas of application within vaccine discovery, preclinical testing, and clinical studies. This month, we conclude with a detailed discussion of specific disease targets and AI’s potential in addressing them. Having highlighted the example of Zika virus in part one, below we focus on malaria, tuberculosis, human immunodeficiency virus (HIV), herpesvirus, hepatitis, and pandemic coronavirus.
Malaria
Malaria is a life-threatening infectious disease caused by several species of the protozoa
Plasmodium (P. falciparum, P. vivax, P. malariae, and P. ovale)
that typically are transmitted by bites of female
Anopheles
mosquitoes. In 2019, the registered number of malaria cases reached 229 million worldwide, with 94% accounted for in Africa. The death rate has slowly declined in recent decades but remains devastating: more tha...
https://www.ziel-gmbh.com
Over the past few years, Oxford Biomedica UK has developed and implemented its fill–finish platform at its 84,000-ft
2
“Oxbox” manufacturing facility constructed in 2019. The first phase of development (45,000 ft
2
) houses four segregated suites for producing bulk viral-vector drug substance (VS) where closed systems and bioburden-control processes apply, and two fill–finish suites for viral-vector drug product (VP) in aseptic processing. The first of the fill and finish suites is expected to be approved in the first half of 2022.
The design includes space for expansion by scale-out as product output demand rises. Segregated suites enable the facility to process different viral vectors simultaneously, including the company’s own LentiVector delivery platform and other types of viral vectors that the company makes through strategic partnerships
as a contract development and manufacturing organization (CDMO).
The processing platform combines a VS pooling stage that applies biobur...
Photo 1:
Cell cultivation for cell-line development
The successful commercialization of a biopharmaceutical product begins with a robust and productive cell line. Inefficient cell-line development (CLD) can lead to costly delays and roadblocks. For that reason, small, new, and virtual companies — and even established and mid-size companies — often seek the support of outsourcing partners to develop their cell lines.
Outsourcing CLD activities can ease many pressures associated with manufacturing new biotherapeutics. The benefits of outsourcing CLD and associated processes include access to specialized expertise and technology:
Access to Specialized Expertise and Technology:
Service partners should provide a high-performing, reliable cell line in a timely manner.
Cost Savings Associated with Building and Maintaining Facilities:
The cost of goods is reduced by preventing the need to spend capital to develop a productive cell line in-house.
Decreased Time-to-Clinic/Time-to-Market:
The use of established ...
The past 40 years have ushered in the most advanced medicines the world has ever seen, with tremendous improvements in biomanufacturing technologies to enable their development. Advances in production technology have brought significant improvements in upstream productivity, which then caused bottlenecks in downstream processing. Although many bottlenecks have been resolved for most biologics, new modalities such as gene therapies and mRNA vaccines are driving the need for differentiated purification solutions. Meanwhile, pressures to increase efficiency and reduce costs continue to mount for all biologics.
Innovative fit-for-purpose purification solutions are essential to the successful expansion of advanced therapeutic modalities beyond niche indications. Astrea Bioseparations is leveraging its expertise in development of customized separation solutions with unique nanofiber technology to bring game-changing purification solutions to market for both traditional and next-generation biologics. Additionall...
The process analytical technology (PAT) and quality by design (QbD) guidelines promoted by the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA) support the idea that quality cannot be “tested into” a biologic product but must instead be part of its process design. Seamless integration of analytical data with bioprocess monitoring and control is crucial to understanding a process and overcoming manufacturing challenges that arise in the course of development.
Monitoring of product quality attributes (PQAs) is a key element of QbD. Protein glycosylation, for one, affects important characteristics such as stability and immunogenicity. Thus, it is considered to be a critical quality attribute (CQA) ensuring the safety and potency of biopharmaceutical products submitted for regulatory approval.
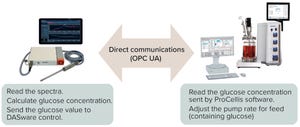
Maintaining steady levels of glucose concentration in production bioreactors is key to control and optimization of process yields and product quality (
1, 2
). Manual bioreactor sampling and...
Inhalable drug formulations show much promise for improving biopharmaceutical delivery. But as Ashleigh Wake (director of business development at Intertek) explained to Ask the Expert attendees in September 2021, such formulations necessitate development of complex quality control (QC) analytical methods. Wake highlighted special considerations for characterizing and controlling the stability and potency of inhaled and nasally delivered biologics.
Wake’s Presentation
Wake emphasized that nasal and inhaled biological products necessitate QC strategies to control both a protein product and its delivery system. Suitable devices and formulations must be selected, and core analytical methods must be established to characterize a drug product’s chemical, physical (e.g., particle size, leak rate, and moisture content), microbiological (sterility), and performance-related (particle or droplet size, spray pattern, and plume geometry) features. Although such methods can be difficult to set up, their requirements ar...
Biomanufacturers traditionally have relied on laminar-flow hoods or thermal tube welding to establish connections for small-format bioprocesses, including those for cell and gene therapy (CGT) production. But such strategies can introduce processing risks and can pose operational challenges. In October 2021, Troy Ostreng (senior product manager at CPC) described how his company’s MicroCNX sterile connectors could meet biomanufacturers’ needs for low-volume applications.
Ostreng’s Presentation
Welding tubes and forging open connections under laminar hoods raise several disadvantages for small-format bioprocesses. Processing speed is important, yet both strategies add procedural complexity and can take several minutes to perform depending on tubing size and type. Hoods and tube-welding equipment occupy valuable cleanroom space, and operators must be trained to use them. They also incur hidden costs for equipment maintenance, validation, repair, and downtime. Ultimately, both connection methods introduce roo...
A 2021 study in the
Journal of the American Medical Association
found that minority groups were underrepresented in US-based vaccine clinical trials, with white people accounting for ~80% of participants (
1
). Although researchers broadly acknowledge that trial representation should mirror the many different populations who could benefit from a drug, lack of diversity continues to hamper clinical-trial effectiveness. To address this problem, the pharmaceutical industry must increase awareness about clinical trials and reduce barriers to participation in them.
Benefits of Diverse Trial Populations
Recruiting diverse trial populations increases healthcare equity. In time, that could alleviate a historic problem: that minorities are “the very same groups disproportionately affected by the diseases being studied in clinical trials” (
2
). Increased representation also enables clinicians to hone their evaluation of treatment outcomes across many different populations. For example, black trial participants t...