with Dr. Sebastian Kleebank
In recent years, single-use bioreactor solutions have been successfully established for animal and human cell culture processes. Such technology is under investigation for microbial applications. Sebastian Kleebank is development and application laboratory manager for the Eppendorf line of bioprocess products. His cell culture and microbial laboratory performs parallel cultivations for prototype testing, user training, and software development.
Kleebank’s Presentation
“The BioBLU 1f bioreactor has a gas-inlet filter for sterile gas supply. It has a fully featured head plate and innovative cooling baffles for high mass transfer rates and cooling capacity. Each vessel has a noninvasive dissolved oxygen (DO) sensor port. The DO sensor does not directly contact culture medium because of a silicone membrane at the bottom. This bioreactor has three Rushton-type impellers, and some versions have two; both use a magnetic drive assembly for stirring. For exhaust condensation, it uses a...
RX-360 CHOOSES AUDIT LEADER
Rx-360, a not-for-profit international consortium representing the world’s leading pharmaceutical and biotech organizations, announced in June a partnership wherein BSI Supply Chain Solutions would lead its international joint audit program. BSI will work with Rx-360 to streamline the audit process and ensure supplier compliance with quality and security standards through verification audits.
Recognizing that globalization complicates quality and security of the supply chain, Rx-360 developed this program to allow multiple companies working with the same business partner to participate in a standardized audit without sacrificing product or process confidentiality. This should reduce audit costs, standardize the audit scope, expand the number of audits conducted, and ultimately reduce supplier audit fatigue while maintaining high-quality standards.
BSI was selected for this engagement following a thorough RFP process involving several other qualified auditing firms. With more th...
The biopharmaceutical industry now incorporates single-use (SU) technology and systems in most production processes based on cell culture (
1
,
2
). Implementation of such technologies has led to the availability of prepackaged and sterilized systems complete and ready for use with preinstalled mixers and monitoring probes. From upstream process- material preparation through final-product formulation, biopharmaceutical sponsors are increasingly presented with numerous SU solutions that support all major production platforms (
3–5
).
GE HEALTHCARE LIFE SCIENCES (WWW.GELIFESCIENCES.COM)
The number of SU materials and suppliers in biopharmaceutical manufacturing has mushroomed since SU systems (SUS) were introduced in the early 1990s. The result of this rapid growth is the commercial availability of many unique SU products, designs, and materials. In turn, such growth has introduced challenges in the establishment of secondary suppliers’ development of component construction, qualification, and validation p...
https://bioprocessintl.com/wp-content/uploads/2014/10/102014_Daniels-Resins.mp3
Single-use manufacturing equipment for the production of certain biological compounds (e.g., recombinant proteins from mammalian cell cultures) makes good sense. Such equipment reduces water and energy use, decreases the need for equipment sterilization and waste treatment, and optimizes space in a manufacturing facility. But consider the plastic resins used to construct the disposable parts of such equipment. In BPI’s April 2014 issue, Tony Kingsbury discussed the
fundamentals of how plastics are made
. In this second installment of BPI’s series on bioprocess plastics, I explain why certain resins are used in single-use manufacturing equipment, how they are made, how their quality is ensured, and whether alternative resins are available readily.
Resins: Natural and Synthetic Polymers
By narrow definition, resins are hydrocarbon secretions of coniferous plants. They are naturally occurring polymers. Examples include amber; pi...
https://bioprocessintl.com/wp-content/uploads/2014/10/102014_Hutchison.mp3
Biopharmaceuticals include recombinant proteins, vaccines, gene therapies, and drug products derived from stem cell technology. One key characteristic of shared by all biologics is that they tend to be extremely large molecules with complex three-dimensional structures, critical to their functionality. For example, monoclonal antibodies (MAbs) are composed of more than one thousand times larger than a molecule of aspirin (one analogy compares the complexity of a MAb to that of an F16 jet, and the complexity of aspirin to that of a bicycle) (
1
).
A single vial of a biological drug product could contain a heterogeneous mix of molecules — all transcribed and translated from the same gene but with subtle differences derived through posttranslational modification processes. For example, Kontroravdi et al. describe how Herceptin (trastuzumab), the MAb treatment for breast cancer, has an N-linked glycosylation profile made up of seven di...
https://bioprocessintl.com/wp-content/uploads/2014/10/102014_specialrpt.mp3
When it comes to biotherapeutics manufacturing, downstream processing groups tend to get “dumped on.” Advances in cell lines, bioreactors, and culture media formulations have increased production output, providing both higher expression titers and greater volumes, but the filters and chromatography columns on the downstream side haven’t kept pace. These century-old technologies haven’t evolved as much and are reaching their limits. Regulatory agencies have contributed to innovation stagnation because they are cautious about manufacturing process changes for fear of undermining quality and consistency of final product. But inconsistency is a hallmark of many current processing methods. For example, chromatography resins can vary in their behavior from batch to batch and degrade over time.
As a drug candidate inches closer to licensure, the pressure mounts on manufacturing. “You’ve got people on one side tweaking the cell culture,” ...
Increasingly efficient bioreactors allow biopharmaceutical manufacturers to achieve higher cell densities. That improved upstream efficiency has led to new purification challenges resulting from high product and contaminant concentrations as well as complex components. Therefore, harvest and clarification techniques are evolving to incorporate feed pretreatment, flocculation, and different filtration technologies such as normal-flow, tangential-flow, and depth filtration. The objective is to increase process capacities and filtrate quality, ultimately reducing biomanufacturing costs.
New strategies for clarification of recombinant proteins (in particular, monoclonal antibodies) and methods of pretreatment can improve clarification efficiency. Such approaches lead to better purity of the obtained filtrate, improving the overall efficiency of downstream purification steps that follow.
Traditional Methods
Using traditional expression systems, bioreactors can support cell densities ranging from 10 to 50 × 10
https://bioprocessintl.com/wp-content/uploads/2014/10/102014_Chamow.mp3
Table 1: Timeline for biotherapeutic MAbs and derivatives, including Fc fusion proteins, approved by the FDA in the indicated years; all are produced using recombinant DNA technology. The protein engineering technology on which each product is based is indicated. Nine of the 45 approved MAb products are Fc fusion proteins.
(
1
First MAb approved under animal-efficacy rule
2
Antibody–drug conjugate
3
Fab/(Fab’)2 fragment)
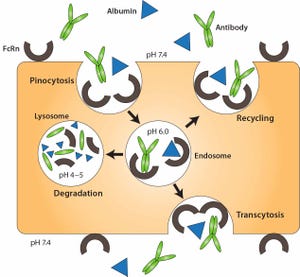
Over the past three decades, 45 monoclonal antibody (MAbs) and MAb-derivative products have been approved for therapeutic use in the United States (Table 1). One class of antibody derivatives is growing in importance: Fc-fusion proteins.
Many biologically active proteins, including receptor ECDs (see “Abbreviations” box), cytokines, enzymes, and bioactive peptides have very short serum half lives because rapid renal clearance limits their exposure in target tissue (and, consequently, their pharmacological effect)...
A Waters Acquity column and an Agilent Q-ToF system were used in this study.
Protein mass is often determined using ultraperformance liquid chromatography (UPLC) coupled with electrospray-ionization mass spectrometry (UPLC/ ESI MS or simply LC-MS). A UPLC system equipped with an ultraviolet (UV) detector serves as an assisting vehicle to deliver purified and separated protein molecules to the mass analyzer. Reserved-phase chromatography (RPC) is the most common chemistry chosen to serve this purpose. For sample purification, not only does RP-UPLC use salt-free mobile phases that are amenable to MS, but it also can efficiently remove buffers, salts, and other sample components that otherwise would interfere with a mass analysis. In addition to sample purification, RP-UPLC has sufficient separation capability to readily separate heavy-chain (HC) and light-chain (LC) antibody domains under reducing conditions (
1
).
However, RP-UPLC/MS has limitations in protein mass analysis. The technology separates protei...
https://bioprocessintl.com/wp-content/uploads/2014/10/102014_Scott.mp3
GRAPHIC STOCK (www.graphicstock.com)
Monoclonal antibody (MAb) production has adopted an accepted technology platform for downstream processing (
1
). The need for more economic processes has been addressed by increasing MAb titers in fermentation and aiming toward greater bioreactor volumes to increase productivity. Consequently, cost pressures are now passed on to downstream process groups. Membrane and chromatography resin savings are more important for MAb processes than ever before, with highly productive cell cultures generating large volumes of process fluid to purify (
2
).
Traditionally, protein A resins have a comparably high share among the costs of consumables in MAb processing. Protein A ligands for chromatography need to be alkaline stable, highly discriminating, with low ligand leaching levels, which requires substantial effort in development of these affinity media. Large feed streams also require higher downstream capa...
https://bioprocessintl.com/wp-content/uploads/2014/10/102014_Stering.mp3
According to current European Union good manufacturing practice (EU GMP), integrity testing of sterilizing-grade product filters should be performed preuse poststerilization (PUPSIT) and immediately after use. In addition, PDA’s Technical Report 26 states that preuse integrity tests are preferably performed after filter sterilization. Performing an integrity test of an already sterilized product filter in-line requires wetting the filter while maintaining the downstream side sterile. The test gas must also be evacuted on the downstream side throughout testing maintaining sterility. The upstream side also must be protected from uncontrolled bioburden, which generally is understood as maintaining sterility of the upstream side by using sterile water for wetting and a sterilizing barrier between the integrity tester and the filter to be tested.
Photo 1: The filter management system (FMS)
The drawback of performing integrity testing on a...
Cord blood is becoming an increasingly popular and important topic of discussion among expectant parents. It comes from a newborn’s umbilical cord and contains hematopoietic stem cells (HSCs), which are the building blocks of a body’s blood and immune system. After a baby is born, cord blood is routinely discarded as medical waste — unless the parents choose to have what blood remains in the umbilical cord collected. Presently, more than 90% of cord blood is discarded, limiting the potential for therapeutic use and additional research.
But there are other options. At some delivery hospitals, cord blood can be donated to a public bank and made available to patients who are in need of stem cell transplants but have no matching related donor available. Expectant parents also might consider storing their baby’s cord blood with a private cord- blood bank. Such companies charge a fee to collect, process, and cryopreserve newborn stem cells for a family’s potential future medical use.
In the United States, more ...