Often I am dismayed to hear local news reporting of early stage clinical milestones for therapies that may still be years in development. I assume that those reporters and news editors receive pretty much the same press releases that we do here at BPI. But I worry that a more general readership hears only the hype, missing details of the process and time required for approval. More than one letter to the editor has opined that “delays” in bringing new therapies to market result more from bureaucratic top-heaviness than necessary controls placed on a drug-development program. Such delays can drive desperate people to treatments in less-regulated regions or to counterfeit therapies altogether — which have for many years included “stem-cell injections” — further complicating relationships between patients and the industry as well as attitudes toward regulation as a concept.
When our local paper (the
Register-Guard
) published news of a breakthrough approved therapy, I was relieved to see that its Associated...
Introducing New Editorial Advisor
Dr. Sanjay Nilapwar works as a purification process scientist in the novel-molecules group of Medimmune in Gaithersburg, MD, where he is also part of the platform chemistry, manufacturing, and controls (CMC) development team for antibody–drug conjugate products. In this position, he looks after the transitioning of ADC and monoclonal antibody (MAb) molecules as candidate drugs from development to the manufacturing stage, which encompasses cycles 1 and 2 purification process development, technology transfer and related CMC aspects including filing of investigational new drug (IND) applications.
In 2013, Nilapwar started working for Spirogen before its takeover by Medimmune. There, he worked on development of site-specific and stochastic conjugations based on pyrrolobenzodiazepines (PBDs) at research scale as well as on stability and analytical assay development. He was instrumental in setting up the medium-scale ADC purification laboratory at Medimmune. He first started wo...
Adobe Stock Image (https://stock.adobe.com)
Patients receiving particulate contamination through parenteral delivery of biopharmaceuticals presents a significant potential health risk. However, the severity of that risk often is unclear. It depends on the route of administration, dosage volume administered, particle properties and amount received, and the ultimate fate of particles within a patient’s body (
1
). The appearance of particulate contamination also can be a visible indicator of product quality. Consequently, when such contamination is discovered within biopharmaceutical manufacturing operations, often it triggers costly investigations and corrective actions.
The bioprocess industry is evolving from widespread stainless steel systems, which are cleaned and steam-sterilized by validated processes immediately before use, toward single-use systems (SUS) that are not routinely cleaned before use. Particles can be generated during SUS fabrication, transportation and assembly. Cleaning and sterilizin...
A space-filling model of allantoin (HTTP://STOCK.ADOBE.COM)
Endotoxin contamination has been the bane of the bioprocessing industry since its inception. Endotoxins are everywhere: They are toxic and/or interfere with every type of therapeutic, diagnostic, and research product; they are indestructible within the limits of product tolerance; and they are difficult to remove (
1
–
4
). Beyond that, they interact with various biological species in ways that prevent accurate measurement (
5
,
6
).
Managing these issues has been a focus of the industry for at least half a century, yet it still too often falls short of manufacturing controls necessary to satisfy regulators (
7
,
8
). It is even more of a problem with diagnostic and research products because their preparation generally involves fewer controls. Development of new endotoxin removal methods thus remains a subject of persistent interest.
Figure 1: Endotoxin basic structure and chemical potentials
Endotoxin removal methods typically focus on particu...
Figure 1: The MiniTEM system is a compact 25-kV transmission electron microscope designed for automated image acquisition and subsequent analysis.
Gene therapy is the transfer of genetic material to a patient’s cells to achieve a therapeutic effect. Therapeutic DNA typically is delivered using a viral vector system, and adenoviruses have been used for this purpose for over 20 years (
1
–
3
). Within the past 10 years or so, lentiviruses have shown promise in clinical trials (
1
–
3
), and adenoassociated viruses (AAVs) have been used in the first approved gene therapies in the Western world (
4
).
The number of gene therapy applications based on viral vectors is growing. Consequently, small-scale manufacturing processes increasingly require scaling up to commercial processes. Because some products have been initially manufactured using very simple concentration and purification methods, scale-up also might include process development.
Viral Vector Manufacturing
Viral vector upstream scale-up challenges in...
The Chinese hamster ovary (CHO) cell line is the most prevalent biopharmaceutical expression system and has been proven safe for commercial production of protein therapeutics (
1
). However, even after multiple purification steps, biopharmaceuticals contain residual host-cell protein (HCP) impurities that pose a potential safety risk to patients (
2
). Health authorities demand close monitoring of HCP impurities and require sensitive analytical methods with high coverage: the ability to detect a broad range of HCP impurities (
3
,
4
).
Polyclonal sandwich immunoassays are used commonly for that purpose because they confer higher sensitivity than other analytical methods (
5
). Pharmacopoeial guidance on HCP assays has been published recently (
6
,
7
). The industry distinguishes three types of HCP immunoassays:
A platform assay confers advantages similar to those of a commercially available assay: It is ready from the onset of product development, makes analytical results comparable across product devel...
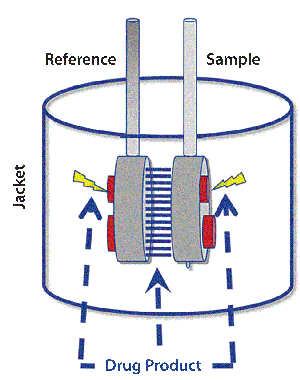
Figure 1: Schematic of the thermal core of a differential scanning calorimetry (DSC) system shows reference and sample cells.
The primary goal of biopharmaceutical process development is to determine what steps and conditions will maximize and optimize yields of purified product in the most reproducible, robust, and cost-efficient way. Characterized by high batch-to-batch comparability minimizing economic losses associated with batch failures, success relies on a thorough understanding of a given biological drug. Determining how its activity and stability are affected by processing and how to mitigate and control associated risks is advocated by a quality by design (QbD) approach. However, the complexity and marginal stability of proteins complicates such determinations.
Forward-thinking companies continuously reevaluate the analytical techniques they apply and appraise new technologies to assess their potential value in helping to maintain a business lead. One such technique currently attracting attentio...
Continuous biomanufacturing was a central topic at the fourth annual World Biological Forum in Oxford, UK, on 26–28 June 2017. A well-rounded lineup of presenters appeared at this forum held in Oxford University’s Lady Margaret Hall, an eclectic location that well captured the historic charm of the university. Delegates were well supported throughout the meeting with generous meals, refreshments, and assistance provided by helpful staff.
Papers were presented in Talbot Hall in the center of the college. The stately main hall building provided such lovely acoustics that no public-address system was required throughout the event. Subi (Ganapathy Subramanian) is an indefatigable organizer and an early believer in the potential of continuous processing as applied to biotechnology. Beyond this, he is a sincere believer in the value of attendees to enjoy robust discourse around the papers presented. He therefore thoughtfully designed activities in delightful gardens and libraries to provide an opportunity for s...
Dave Howes (senior applications specialist at ILC Dover) introduces his company’s market-leading solution for single-use powder containment.
Howes’s Presentation
Some challenges in biopharmaceutical powder manufacturing include optimizing open-suite facilities; minimizing product loss from spills; lessening waste and cross-contamination; reducing worker exposure to airborne particulates; and lowering the risk of ignition with flammable materials.
Characteristics to look for in a powder-transfer system are
The EZ BioPac system provides an answer to meeting the challenges above with all needed characteristics. It is designed specifically for powder transfer and containment by ILC Dover experts in this field. The design addresses the issues highlighted above and offers distinct advantages over systems that have been derived from liquid-handling technology. The EZ BioPac product range covers 1–100 L, with flange sizes of 1.5–8.0 inches.
Filling and Discharging:
An EZ BioPac system offers multiple advantages ...
Tony Fultz (director of upstream manufacturing at Fujifilm Diosynth Biotechnologies) presented an “Ask the Expert” webinar on 23 August 2017.
Fultz’s Presentation
Human error was responsible for about 75% of batch failures at contract manufacturing organization Fujifilm Diosynth Biotechnologies in 2016. Technicians operate in a dynamic and stressful environment, both contributing factors to human error. With multiple projects running concurrently, some employees are involved in executing several processes during a shift.
The company has several ways of reducing human error: e.g., human-performance tools, a certification program dependent on theory-based training, and a robust method of task performance. Proper task performance is a three-step process using human-performance tools to provide a consistent approach to all tasks, regardless of their complexity: Discuss it, do it, review it (DDR).
Discuss It:
The first step ensures that a performer and a witness identify their roles and share an understanding...
On 6 September 2017, Mark Snyder (manager of the process chromatography R&D
applications group at Bio-Rad Laboratories) gave an “Ask the Expert” presentation on process-scale column packing with CHT ceramic hydroxyapatite media.
Snyder’s Presentation
CHT is an incompressible mixed-mode chromatography medium using cation exchange and calcium-affinity interactions, and it is available in 40-μm and 80-μm particle sizes. This medium can be used to purify monoclonal, polyclonal, and bispecific antibodies; antibody fragments; other recombinant proteins and isozymes; viruses, viral particles, and vaccines; and supercoiled, single-stranded, or double-stranded DNA. It is particularly good at viral clearance and separating nucleic acids.
The nominal packing density of CHT media is about 0.63 g/mL of packed bed. This value is determined by placing a known mass of the powdered medium into a graduated cylinder, tapping the cylinder several hundred times, and then measuring the volume.
For real-world packing, however,...