WWW.LIFETOUCH.COM
As we send this issue to the printer, the BPI editors are enjoying the start of the western Oregon fall season. Early rains are beginning finally to extinguish the remaining wildfires that have kept our valley (along with much of the west coast) choked with smoke much of the summer. With the fires on this side of the country and the recent storms to the east, we hope that all our colleagues and readers remain safe.
For BPI, changes begun this year will continue in its 17th year of publication as we integrate ourselves more purposefully into Informa’s KNect365 division. Some of you might remember that the magazine was founded within the IBC conference organization. We always have worked closely with our conference organizers. But in publishing (as in the biopharmaceutical industry), technology platforms can be key to successful collaborations. Now we are benefiting from improved technologies for sharing content and creating projects together. After all, both editors and conference organiz...
Launched in June 2018, the BioProcess Insider digital information portal delivers the latest financial and business news and expert insider views influencing the commercialization of biopharmaceuticals. Here are just a few recent stories edited for our space limitations in print. For more discussion and in-depth analysis, check out the website at
www.bioprocessinsider.com
. Every edition provides expert and insider perspectives on current financial movements and deal-making; the newest technology purchases and capacity investments; regulations affecting the bioprocessing sector; global market actions and reactions; and industry trends.
Originators Rebuff Pfizer Claims of Misleading Biosimilar Talk
Accusing Amgen, Johnson & Johnson (J&J), and Roche of sending misleading communications about biosimilars, Pfizer is asking the US FDA to issue guidance on such behavior. In a Citizen Petition published on 22 August 2018, Pfizer said that communication tools intended to incentivize the adoption of and switching...
Single-use (SU) technology is used more every year throughout the biotechnology industry. As applications now span from cell banking to drug product, that in turn is raising interest in the interaction of extractables with proteins and cells. The second “Single-Use Technologies” conference organized through Engineering Conference International (ECI) was subtitled “Bridging Polymer Science to Biotechnology Applications” and delved into the science of plastics in bioprocessing applications. On 7–10 May 2017 at the Hotel dos Templários in Tomar, Portugal, people from many job functions gathered to share their issues, understanding, and solutions.
The conference was well attended by representatives from end-user organizations; academia; and suppliers of resin, film, single-use components, and gamma irradiation solutions. They participated in positive, scientific, collaborative discussions among different functional areas. This meeting covered a few topics in greater depth that had been addressed at the first ...
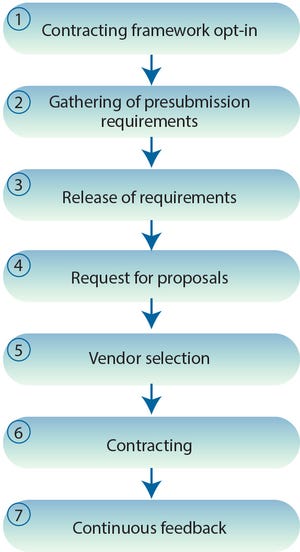
Proposing a Systematic QbD Approach Toward Validated Guidelines for CMO RFI and RFP Processes: Biopharmaceutical Vendor Evaluation and Selection Minimum Standards (BioVesel)Proposing a Systematic QbD Approach Toward Validated Guidelines for CMO RFI and RFP Processes: Biopharmaceutical Vendor Evaluation and Selection Minimum Standards (BioVesel)
Figure 1: Seven steps proposed by BioVesel for procuring CMO services
Three major concerns predominate biotechnology executive management in organizations of all sizes and above all other risks: finance (or its absence at critical moments), technological performance, and failures in coordination. Some business functions, such as human resources (HR), are effectively siloed horizontally and therein are more likely to be susceptible to only one of those risks (
1
). Few functions are subject to this trinity of risks simultaneously; all functions may be exposed to failures in internal coordination, and a smaller subset can be prey to challenges of both internal and external coordination. But manufacturing can be exposed to all three concerns (
2
).
Biotechnology companies are vertically integrated by nature – and particularly companies pursuing contemporary novel biological therapeutic platforms such as cell, gene, and viral therapies (
3
). They operate within a predominantly virtual business model by outso...
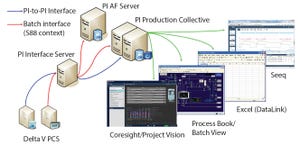
Figure 1: BMS clinical active pharmaceutical ingredient (API) manufacturing scale up: PI infrastructure (Delta V PCS from Emerson), Seeq (Seeq Corporation), Excel (Microsoft), and ProcessBook and Coresight/Project Vision (OSIsoft)
With all the hype surrounding the industrial Internet of Things (IoT), cloud computing, and digital transformations, the most important information technology factors still are data and the connections of sensors, systems, and applications that generate, store, find, and use those data to obtain operational intelligence. Data volumes are increasing rapidly, and they will continue to do so. The ability to find and make sense of data to obtain intelligence that improves process outcomes is more important than ever.
For clinical- and commercial-scale manufacturing in the pharmaceutical and biotechnology industries, just finding the right data to analyze can consume significantly more time than it does to perform analyses. Having the right applications to store and contextualize dat...
Vero cells infected with purified influenza A: Infectious virus particles were stained with rabbit antiinfluenza A NP-antibody and a goat antirabbit IgG antibody conjugated with AlexaFluor 488 (green). Cell nuclei are stained blue with DAPI (4‘,6-diamidino-2-phenylindole).
Influenza is a global respiratory disease with an estimated mortality of up to a half million people per year (
1
). The majority of traditional influenza vaccines are still produced in eggs. Downstream processing typically consists of clarification by centrifugation, concentration by ultrafiltration, and purification by ultracentrifugation (
2
).
Recombinant vaccines are most often purified by chromatography. Chromatographic purification of viruses already has achieved major improvements in recovery and scalability (
3
), but it also is important because it enables virus purification to keep pace with important regulatory and manufacturing trends across the field of biopharmaceuticals. One of those trends is
process intensification
, ...
WWW.ISTOCKPHOTO.COM
Biotherapeutics from recombinant DNA technology include diverse modalities such as peptides; enzymes, antibodies, and other proteins; nucleic acids; and cellular therapies. Such products present physical, chemical, and biophysical challenges.
Excipients used in stabilization of these biotherapeutics can be broadly classified into the following classes (subgroups) that have been reviewed carefully elsewhere (
1
–
4
) :
Sugars and polyols form an important subgroup of excipients used to stabilize biotherapeutics, and their use has been reviewed in several important publications (
5
–
9
). Disaccharide sugars (e.g., sucrose, trehalose, maltose, and lactose) and polyols (e.g., mannitol, sorbitol, and glycerol) all find applications in biotherapeutic stabilization. My goal herein is to review their use for biotherapeutic stabilization and provide a user’s perspective on how to select these excipients rationally depending upon properties of a given molecule and its stage in biopharmaceutical...
WWW.GETTYIMAGES.COM
A general vaccine purification strategy can be divided into three stages, with one or more steps for each stage. The first stage is to concentrate and isolate the target molecule quickly to remove it from conditions that could lead to its inactivation or loss. Intermediate purification seeks to remove remaining contaminants, typically using an orthogonal approach. That is followed by a polishing step in which trace impurities are removed through high-efficiency steps because those impurities usually are similar to the target molecules.
Chromatography is a strong tool and a scalable method for achieving maximum purity in vaccine downstream processing and contaminants/impurities removal (
1
). Other common purification methods include precipitation, ultracentrifugation, tangential-flow filtration (TFF), and enzymatic digestion (
2
,
3
). Chromatography benefits manufacturing through step reduction (
4
), higher yield, and faster processing (
5
). Charged depth and membrane filters (
6
)...
The therapeutic benefits of monoclonal antibodies (MAbs) have been demonstrated in recent decades with uncontestable success as treatments for human disease. Despite MAbs’ key features such as specificity, selectivity, and safety, the format has limitations (
1
,
2
). Bispecific antibodies may overcome number of difficulties (
3
).
Multiple formats of bispecific antibodies have been developed, although only the κλ-body is fully human and devoid of linkers or mutations. It requires no genetic modifications of heavy and light chains and results in bispecific antibodies with natural sequences (
4
). Different affinity chromatography steps have been developed for purification of bispecific MAbs.
However, development of a nonaffinity–based platform leads to more cost-effective production processes. The advent of hydrophobic cation-exchange resins, often referred to as
mixed mode
, provides opportunities to reduce the number of affinity steps in a platform process. Establishing protocols for such chromatograp...
Experimental set-up for
k
L
a
value determination
In recent years the biopharmaceutical industry has significantly increased the demands it makes on bioreactor systems. Efficient and reproducible production of active pharmaceuticals of high quality and in large quantities is of highest priority. However, bioprocessing is a complex topic. Numerous factors affect growth of cells in culture but are difficult to determine and interpret reliably. One of the most relevant performance parameters is the volumetric mass transfer coefficient (
k
L
a
). It describes the efficiency of gas transfer (e.g., oxygen) from a gaseous phase to liquid solution, which is important because living cells can metabolize oxygen only when it’s dissolved in solution. To allow for cell metabolism to function at its fastest, the dissolved oxygen (DO) concentration must be above a critical level at any point within a bioreactor.
The
k
L
a
value is influenced by a number of factors. They include process parameters (agitator speed, te...
Bio-Rad’s CHT Ceramic Hydroxyapatite XT (CHT XT) calcium-affinity cation-exchange chromatography medium is used for purifying numerous types of biomolecules, with single-step clearance of impurities and aggregates in monoclonal antibody (MAb) purification. This is an easy-to-use mixed-mode medium, which is both ligand and support matrix in one. In an “Ask the Expert” webinar on 18 July 2018, Mark Snyder (manager of the process chromatography R&D applications group at Bio-Rad Laboratories) discussed its application and use at process scale, providing guidelines on where to begin in selecting hydroxyapatite media.
Snyder’s Presentation
Ceramic Hydroxyapatite (CHT) is a crystalline mineral of calcium and phosphate, Ca
10
(PO
4
)
6
(OH)
2
. In CHT chromatography media, ligand and matrix are the same. This mixed-mode medium works as a universal polishing matrix through both calcium affinity and cation exchange. It is unequaled in clearing a full range of impurities: aggregates, protein A, host-cell proteins, ...
On 12 September 2018, Thomas Page, PhD (vice president of engineering and asset development at Fujifilm Diosynth Biotechnologies, FDB) shared a brief overview of challenges encountered in manufacturing of advanced biotherapeutics.
Page’s Presentation
FDB is a contract and development manufacturing organization (CDMO) that uses microbial and mammalian cell lines to produce protein therapeutics as well as viral vaccines and gene therapies. The company’s Center of Excellence for Advance Therapies Manufacturing is located in Texas. FDB set out some years ago to make sure that it could cover the path from development through clinical manufacturing into process validation and commercial manufacturing.
Page discussed key points to consider when working with advanced therapies. He began with the product life cycle, focusing on early phase development, when sponsors should have an understanding of their own phasing. Many start with a proof-of-concept for their construct that might be at odds with long-term needs. ...
The transition from transient to stable production workflow with Chinese hamster ovary (CHO) cells requires a change in culture medium. On 22 August 2018, Shreya Lowmaster (field applications scientist at Thermo Fisher Scientific) discussed transient to stable solutions for moving from research to commercial production.
Lowmaster’s Presentation
Researchers are demanding higher titers in transient systems, and many people use CHO systems as an alternative to HEK293 cells. The Thermo Fisher Gibco ExpiCHO Expression System kit was developed for transient expressing pools used in research. Now the company introduces Gibco ExpiCHO Stable Production Medium for commercial-scale production. These two products are integrated into one system.
In the transition, users typically need to screen and optimize media. Normally, a medium would be optimized for the cloning process and then adapted for manufacturing. It can take over three months to go through that process. With Gibco ExpiCHO Stable Production Medium, you c...
Until relatively recently, life-science research was characterized by test tubes, Petri dishes, and centrifuges. Now, as with many industries, the life sciences are undergoing a digital transformation.
Computational science is changing laboratory design. The healthcare industries always have generated large amounts of data. What has changed is the available information technology. With the growth of cloud computing, large data sets — and the high-speed tools for analyzing them — are available increasingly to a degree not possible with traditional servers and desktop computers. For example, one researcher can sequence 18,000 human-genome base pairs and generate about 1.8 petabytes of data, the equivalent of 9,000 hard drives.
To better understand treatment efficacy, life-science organizations now can access real-world data from a host of sources: e.g., medical insurance claims, genomic research, electronic medical records, and clinical trials. With artificial intelligence and machine learning, companies ca...


schedl_b_and_w.jpg?width=100&auto=webp&quality=80&disable=upscale)