Rational Selection of Sugars for Biotherapeutic Stabilization: A Practitioner’s PerspectiveRational Selection of Sugars for Biotherapeutic Stabilization: A Practitioner’s Perspective
October 15, 2018

WWW.ISTOCKPHOTO.COM
Biotherapeutics from recombinant DNA technology include diverse modalities such as peptides; enzymes, antibodies, and other proteins; nucleic acids; and cellular therapies. Such products present physical, chemical, and biophysical challenges.
Excipients used in stabilization of these biotherapeutics can be broadly classified into the following classes (subgroups) that have been reviewed carefully elsewhere (1–4) :
Buffers (e.g., phosphate, acetate, and histidine)
Tonicity agents/stabilizers (sugars such as sucrose, polyols such as sorbitol)
Bulking agents (lyoprotectants such as mannitol)
Surfactants (e.g., polysorbates)
Antioxidants (e.g., methionine)
Metal ions/chelating agents (e.g., ethylenediaminetetraacetic acid, EDTA)
Preservatives (e.g., benzyl alcohol).
Sugars and polyols form an important subgroup of excipients used to stabilize biotherapeutics, and their use has been reviewed in several important publications (5–9). Disaccharide sugars (e.g., sucrose, trehalose, maltose, and lactose) and polyols (e.g., mannitol, sorbitol, and glycerol) all find applications in biotherapeutic stabilization. My goal herein is to review their use for biotherapeutic stabilization and provide a user’s perspective on how to select these excipients rationally depending upon properties of a given molecule and its stage in biopharmaceutical development.
Before optimizing a formulation and rationally selecting stabilizing excipients, formulators must consider thoroughly a product’s therapeutic indication, dose, route of administration (intravenous or subcutaneous administration), stage of development (e.g., early clinical trials or late-stage commercial), commercial presentation (e.g., in a vial or low-volume prefilled syringe) and so on. Other concerns include the availability of highly pure excipients. Trace-metal, inorganic, and organic impurities are a critical aspect if a biomolecule is sensitive to them. Residual endotoxin levels in excipients, particularly those of natural origin, also are an important consideration. Other aspects such as price and availability of multicompendial excipients and a robust supply chain that will not be susceptible to delays also influence the choice of excipient.
Stabilization Mechanisms
Qualifying: Sugars have been used for decades to stabilize proteins, and it is important to understand the overall mechanisms by which they do so. Professor Serge Timasheff described the protein stabilization mechanism of sugars and polyols in the 1980s (10–14). Preferential interaction/exclusion is the mechanism by which polyols stabilize the native structure of proteins and increases the free energy of unfolding, thus thermodynamically favoring the native folded state. The phenomenon also prevents interaction of proteins with solvents.
Ohtake et al. summarized parallel theories of stabilization mechanisms, including surface tension, the excluded volume effect, and interactions with peptide bonds (5).
Several recent studies on the stabilization effects of sugars and polyols have been published, some even presenting a mechanistic understanding. Nicoud et al. (15) discuss polyol sugars in stabilization of monoclonal antibodies (MAbs), suggesting that the effect does not depend solely on the volume fraction occupied by a polyol but also its chemistry. Moreover, the stabilization effect at a given polyol concentration is polyol specific, increasing as a function of size. Polyols with six carbon atoms or more have a similar stabilization effect. Kaushik and Bhat evaluated the exceptional stabilization effect of trehalose on thermal stability of five well-characterized proteins (16), mostly enzymes in aqueous solutions but differing in their physicochemical properties.
Quantifying: Once the general mechanism of stabilization is characterized and understood, we can ask how much stabilizer is enough.
Cleland et al. describe a study addressing the question for storage stability of a lyophilized antibody (rhuMAb HER2) formulated with either trehalose, sucrose, or mannitol (17). They found a 360:1 molar ratio of lyoprotectant (trehalose, sucrose, or mannitol) to protein to be necessary and reported that the sugar concentration was three- to fourfold below the isoosmotic concentration typically used in formulations. The 360:1 molar ratio is 60 mM sugar concentration for a protein concentration of 25 mg/mL. Formulations combining sucrose or trehalose (both at 20 mM) with mannitol (40 mM) were comparably stable to those using sucrose or trehalose alone at 60 mM concentration. Note that the commercial rhuMAb HER2 product (trastuzumab) is formulated with 60 mM trehalose probably because it has a higher glass-transition temperature (Tg) than sucrose and thus allows energy-efficient lyophilization (18).
The 360:1 molar ratio for trehalose and rhuMAb HER 2 translates to a ~1:1 weight/weight ratio. Hence, for proteins stabilized by sugars, we typically observe a sugar/protein weight ratio of at least 1:1 (19). If a formulation is designed for subcutaneous administration, then the amount of sugar added serves the dual purposes: stabilization and osmolality adjustment. The latter is critical for subcutaneously administered products because differences in osmolality and use of certain excipients often are related to pain during injection (20–22). Biologics for subcutaneous administration typically are restricted in their total administered volume (<1.5 mL) and generally are formulated at an osmolality of ~300 mOsmo/kg to prevent patient pain (23). The 120 mg/mL trastuzumab liquid formulation for subcutaneous administration uses 210 mM trehalose dihydrate (~80 mg/mL) as a stabilizer. In this case, the weight ratio of trehalose to protein is under 1:1. The difference highlights that the ratio of sugar stabilizer to protein is selected case by case and varies significantly depending upon protein concentration and final dosage form — e.g., lyophilized solids or liquid compositions (24).
Barnett et al. evaluated the effects of common osmolytes and stabilizers on MAb stability (25). They measured Kirkwood–Buff integrals for both protein–water (G12) and protein– osmolyte interactions (G23) as a function of osmolyte concentration. The density of antistreptavidin immunoglobulin gamma-1 (AS-IgG1) in ternary aqueous solutions (water–osmolyte–antibody) was measured for sucrose, trehalose, sorbitol, and polyethylene glycol (PEG), respectively. Of the tested osmolytes, sucrose and PEG change from being preferentially excluded to preferentially accumulated with increasing concentration. This study again highlights the significance of measuring such interaction parameters for each therapeutic protein and rationally selecting stabilizers case by case.
Several researchers have explored identifying the protein stabilization mechanism for sugars in a dry (solid) state. Chang et al. evaluated the stabilization mechanism of an IgG1 antibody (150 kD) and recombinant human serum albumin (rHSA) (65 kD) when formulated with sorbitol, trehalose, and sucrose (26). Sorbitol offered comparatively minimal protection against aggregation and chemical degradation. These findings suggest that both chemical degradation and aggregation rates of the protein decreased when the sucrose/protein weight ratio increased from 0 to 2:1. Fourier-transform infrared (FTIR) spectroscopy findings suggested that IgG1 storage stability improved when the native structure of protein was preserved during drying.
Han et al. evaluated sucrose and trehalose as stabilizers for rHSA both alone and combined with mannitol. Lyophilized formulations of rHSA with either sucrose or trehalose and combined with mannitol were subject to storage at 35 °C. The battery of tests included sodium-dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and structural characterization tools: differential scanning calorimetry (DSC) and FTIR. Both sucrose and trehalose remained amorphous during freeze-drying, whereas mannitol remained partially amorphous (27). These findings identify sucrose and trehalose as effective stabilizers for rHSA protein that maintain its stability in the solid state during storage.
The distinct stabilization effects of sucrose and trehalose on stability of lysozyme and myoglobin formulations processed by supercritical fluid drying and freeze-drying also have been evaluated (28). Analyses of both formulations determined the impact of the sugar types and drying techniques. Sugars act as stabilizers by maintaining the native secondary and tertiary structure of the protein intact in an amorphous state during freeze-drying and long-term storage, and the significance of formulation Tg has been well studied. Duddu and Dal Monte compared trehalose and sucrose as stabilizers for a chimeric therapeutic antibody. Accelerated storage stability for both formulations was evaluated at 60 °C, and trehalose (Tg ~80 °C) was found to perform better than sucrose (Tg ~59 °C). The findings also suggest that accelerated stability data generated from glassy-state formulations (40 °C) are better predictors of the relative formulation stability than an extremely high temperature of 60 °C — at which sucrose (Tg ~59 °C) doesn’t remain in a glassy state and thus is not a good stabilizer (29). Other important reviews have summarized protein stabilization mechanisms by sugars during freezing and in the solid state (30–32).
The Role of Formulation Development
Formulation and stabilization-related activities happen at different stages of biopharmaceutical development. Understanding the role of formulation development provides an insight into why and how certain excipients are preferred. In summary, formulation development is a service function catering to biotherapeutic development groups to deliver safe and stable products. Formulation development covers the entire range of related activities, certain sets of which are undertaken at each stage of product development.
Early Stage Discovery, Candidate Selection, and Developability Assessment: Early stage discovery and development activities focus on candidate selection, determining key biophysical and biochemical properties of a biomolecule, and developing an overall mechanistic understanding of its physicochemical instability. The excipient selection strategy during this early stage is fairly liberal: All generally regarded as safe (GRAS) sugars are screened — disaccharides (maltose, sucrose, trehalose) and polyols (mannitol and sorbitol) — as prototype formulations are developed, and their long-term stability is evaluated. High-throughput (HT) biophysical screening tools help formulators assess developability of biomolecule product candidates (33–42). Platform approaches help the teams come up with prototype formulations that can be optimized further for early stage clinical materials (43–45). Development studies at this stage also include a forced-degradation component that enables identification of breakdown products and helps scientists assess the inherent ability of a formulation to protect a biomolecule against stressful conditions.
Each excipient used for screening and biotherapeutic stabilization has a unique set of physicochemcial properties that a formulator should be aware of. For example, mannitol is widely known to crystalize and perturb protein secondary and tertiary structure during freezing (46–53). Hence, if a product will be frozen for long-term storage, then physicomenchial properties of mannitol would have to be thoroughly evaluated before use as a stabilizer. Its crystallization behavior is not prohibitive to biotherapeutic stabilization, and in several instances mannitol has been used successfully as a stabilizer in liquid formulations (e.g., adalimumab). In such cases, its physicochemical properties and behavior are modified by adding an amorphous sugar costabilizer (e.g., sucrose) (54), adding other glassy substances that in most cases can be the protein itself (55, 56), or even adding other stabilizers such as salt (51).
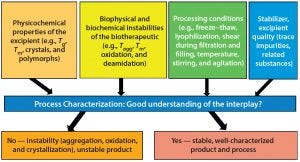
Figure 1: Interplay among excipient, biotherapeutic, and processing conditions — the key to successful formulation and stabilization of biotherapeutics
Figure 1 illustrates the interplay between the physicochemical properties and quality of an excipient, biochemical and biophysical instabilities of biotherapeutics, and processing condition(s) used during formulation and product manufacturing. Thorough understanding of this interplay is necessary for successful formulation development and biotherapeutic stabilization.
A careful survey of recently approved antibody-based products suggests that a histidine buffer (pH 5.5–6.5), a disaccharide stabilizer (trehalose or sucrose), and a surfactant (polysorbate 20 or 80) can provide a good platform formulation composition (44). Once such a prototype platform formulation composition is identified, samplesparing HT approaches can be used to identify the most stabilizing composition and optimize the concentration of each stabilizing excipient. The “go/no-go” decision on a candidate biomolecule is an output at an early stage in development, so degradation mechanisms such as oxidation, deamidation, and aggregation should have been investigated thoroughly by this point. Different stabilization strategies can be adopted for each of those. Oxidation of methionine sites can be controlled by adding chelating agents or free methionine (which gets preferentially oxidized) to the formulation. Deamidation can be controlled either by optimizing formulation pH or replacing asparagine (Asn) residues with other amino acids — or sometimes even by converting a liquid formulation into a solid product (frozen, freeze dried, or spray dried).
Wakankar et al. discuss the effect of glycerol and sucrose cosolutes on isomerization of aspartic-acid (Asp) residues and conformational stability in a MAb (57). Although glycerol or sucrose will increase the overall conformational stability, the Asp 32 isomerization increased significantly in the presence of both cosolutes. Findings suggests that the general stabilization approach merely focusing on increasing conformational stability might not be enough for cases in which chemical instability is a major degradation pathway. Results also indicate that every molecule is unique, so degradation pathways need to be investigated and a stabilization approach rationalized case by case before selecting platform-based approaches.
A quick literature review reveals that each sugar stabilizer (sucrose, trehalose, mannitol, sorbitol, glucose, and lactose) has been studied extensively for its stabilization effect with several different biotherapeutic modalities: antivenoms, protein-based vaccines, peptides, fusion proteins, and antibodies (all isoforms). All such modalities have unique biochemical and biophysical properties. Although some or all of those sugars work as excellent stabilizers for given modalities, it is widely acknowledged that because each biotherapeutic presents unique formulation challenges, formulation development must be case by case. Because biotherapeutic material availability often is limited during early stages of product development, the general industrial approach is to try a stabilizer that works best for most molecules, then optimize the composition to address specific instabilities. Identifying instability mechanisms, a set of optimal stabilizers, and well-defined strategies to develop an optimal formulation are often deliverables from this early stage in development.
Know Your Molecule |
|---|
Practitioners are encouraged to answer some fundamental questions regarding biotherapeutics under study and in development: |
Are structure and stability well understood? |
For first-in-class drugs, are secondary and tertiary structure well characterized? |
Is the mechanism of action (MoA) well characterized and involved/necessary structural components identified? |
Are instability mechanisms known? Does instability also affect the MoA — and hence potency (efficacy) or safety? |
Are both biochemical and biophysical instability mechanisms well characterized? |
Do trace elemental impurities in excipients influence aggregation and biochemical degradation? |
Is the biomolecule susceptible to freeze–thaw processing? |
Clinical Development: Once prototype formulations are developed, the activities that follow generally support clinical evaluation of a candidate until product approval and registration. These activities include developing processes for manufacture of a drug substance (DS) and drug product (DP), designing studies to ship material, performing fundamental studies to address high-dose (highly concentrated) formulation development, and running stability studies to support clinical use of a product.
Early Stage Clinical: During early stages of clinical development, the aim is to develop a DP for first-in-human (FIH) studies. Lyophilized DP has been a popular choice here, but developing a stable lyophilized product can be tricky and time consuming (58). Several discussion forums are debating whether a frozen DP could provide an equivalent alternative (59).
Developing frozen solutions or lyophilized products brings up the question of freezing-related instability of a biotherapeutic during freeze–thaw processing and the behavior of sugars during such processing. These important issues have been addressed in several studies and reviews (60–71). It should be noted that several of those studies focus on characterization of trehalose crystallization during freeze–thaw processing (72, 73). Formulators should be aware that certain sugars (mannitol, trehalose, and sorbitol) crystallize during freeze–thaw processing. Furthermore, sucrose is the only known sugar that remains amorphous during freezing and thawing. Also, formulation composition (buffers, sugars, and protein content), processing temperature, and containers (both volume and shape) affect the freeze–thaw processes for biologics.
Crystallization of sugars is an important consideration and must be addressed during early stages of development. This ensures that stabilization, feasibility, stabilization, feasibility, scale-up, and development studies are performed early in a program. Such studies and large-scale freeze–thaw processing equipment ensure that a robust process is available for clinical manufacturing. Considerations regarding appropriate choice of freeze–thaw systems for biomanufacturing could require additional capital and add delays to overall program timelines.
Availability of multicompendial excipients “just in time” is another aspect to consider for early stage clinical manufacturing. Multicompendial high-purity sugars such as glucose, fructose, maltose, sucrose, trehalose, and mannitol are available, but other atypical stabilizer choices must be evaluated thoroughly. Using such excipients could incur additional cost; cause significant delays in supply; and often will require custom processes for biosynthesis, purification, and quality control.
Stability of a biotherapeutic during patient administration is another important consideration during early stage clinical development. Besides identifying potential incompatibilities and instability, such studies help identify the best conditions for administering a product during clinical evaluation. Trastuzumab DP has a known incompatibility with the 5% dextrose typically used for intravenous administration. Several published studies describe methods to identify such incompatibilities and formation of subvisible particles (74–76). Besides those particles, glycation of antibody-based products is another concern that should be addressed if dilution is performed in reducing-sugar solutions such as dextrose (77). Glycation of a recombinant monoclonal IgG2 antibody has been reported with sucrose formulations at 37 °C after just a month. Note that no glycation was observed when the formulation was incubated at 4 °C over 18 months (78).
Intermediate Clinical Evaluation: As a candidate biotherapeutic moves into clinical development, typical formulation studies support
development of formulations with multiple strengths for dose adjustment (dose-finding studies)
development of product for an alternative route of administration (e.g., subcutaneous administration for a drug initially administered intravenously)
manufacturing processes for clinical drug supply.
Typical development activities undertaken during intermediate clinical evaluations include process development studies to support manufacturing process scale-up, characterization of a bulk freeze–thaw process, shipping studies to determine stability of formulated DS and DP, and generation of comprehensive stability data on both DS and DP. Specific instabilities in clinical formulation (if any) are addressed and their root causes analyzed at this stage.
Weinbuch et al. discuss the presence of nanoparticulate impurities in pharmaceutical-grade sugars and their interference with light-scattering analyses (79). Identifying such impurities in sugars is important if a formulation demonstrates instability characterized by a high subvisible and nanometer-sized particulate impurities. Such impurities are suspected to be immunogenic and thus a topic of recent discussions with regulatory agencies (80). A second concern is the presence of elemental impurities in sugars. Wang et al. have reviewed the impact of residual impurities on protein stability (81). Trace-metal impurities catalyze some instability reactions, particularly those related to oxidation and disulfide-bond cleavage (82). Most biotherapeutics are susceptible to breakdown following such reactions, hence controlling elemental impurities is recommended for pharmaceutical excipients and has been much discussed recently (83).
Clinical manufacturing processes often undergo scale-up and optimization during the intermediate stage. Freeze–thaw processes (scale, volume, and containers) generally are optimized as well. Biologics formulations typically composed of a protein, buffer, and stabilizers (sugars, amino acids, and surfactants) undergo cryoconcentration during freezing. That process generally is characterized and its implications on product stability understood earlier in development (64–71).
With highly concentrated formulations for subcutaneous administration, development studies must characterize a biomolecule’s high-concentration behavior, elucidate viscosity effects and injectability properties of its formulation, and develop processes and methods for ultrafiltration and final formulation. Formulation compositions influence ultrafiltration/diafiltration (UF/DF) processes (84). The Donnan and volume exclusion effects have been investigated in particular for excipient-concentration changes in retentate of buffered biotherapeutic solutions undergoing UF/DF (85, 86). Buffer composition in retentate — particularly for charged and uncharged small-molecule excipients (e.g., histidine, sucrose) — should be verified after UF/DF. Miao et al. describe a theoretical model for predicting such concentrations in solutions of therapeutic antibodies undergoing UF/DF (87).
Late-Stage Development: As a biotherapeutic progresses through clinical testing and meets the required endpoints, chemistry, manufacturing, and control (CMC) efforts move toward demonstrating consistent manufacture, process performance and qualification (PPQ), process characterization (PC), and process validation (PV). In the era of quality by design (QbD), typical outcomes include defining a formulation design space, performing robustness studies, and outlining a control strategy for manufacturing.
No specific excipient-related activities are undertaken during this late-stage development. Rationally selected quality excipients from earlier stages help improve implementation of QbD principles and development of robust biomanufacturing processes. Developing a consistent supply of quality excipients for global manufacturing and distribution of biotherapeutics is another important area of work during later clinical stages.
Life-Cycle Management of Biotherapeutic Products: Formulation development and excipients play a significant role in biotherapeutics life-cycle management. With several marketed products, a second-generation product has been developed and commercialized eventually for life-cycle management and ensuring consistent market share.
One attractive strategy is developing liquid formulations of products initially marketed in lyophilized form. Two commercial examples are abatacept and trastuzumab, for which a “lyo-to-liquid” formulation change was implemented. Excipient choices are extremely critical in such instances.
For abatacept liquid formulation, the stabilizing sugar is sucrose (lyophilized product is stabilized using maltose). The other formulation ingredients include phosphate buffer and polaxomer. Sucrose is preferred for the liquid product probably because it is highly soluble, so the liquid solution can be frozen easily as formulated DS, and sucrose remains amorphous during freeze–thaw processing and storage.
For the trastuzumab liquid formulation for subcutaneous administration, the buffer (histidine), sugar (trehalose), and surfactant (polysorbate 20) are all similar (though not at equivalent quantities) to those in the lyophilized product. The liquid formulation also contains methionine and a proprietary hyaluronidase enzyme (rHuPH20). The methionine probably acts as an antioxidant, and the hyaluronidase facilitates subcutaneous administration of large volumes. When liquid formulations use the same excipients as those used for lyophilized products, historic stability information and compatibility information can be leveraged for the second-generation product review and approval. A formulation change would require significant scientific understanding of product stability and compatibility, and some modifications to the final stages of DP manufacturing would be required. Also, if the route of administration changes (e.g., subcutaneous instead of intravenous), then additional pharmacokinetic studies and a comprehensive understanding of drug distribution would be required.
Developing a highly concentrated low-volume injection for subcutaneous administration is another attractive life-cycle management strategy. A 120-mg/mL liquid formulation of rituximab for subcutaneous administration is commercialized and available in vials (1,400 mg/vial containing 11.7 mL product). The product contains recombinant antibody, human hyaluronidase (rHuPH20), l-histidine, l-histidine hydrochloride monohydrate, α-trehalose dihydrate, l-methionine, and polysorbate 80. This formulation is distinctly different from the firstgeneration rituximab product for intravenous infusion.
A similar second-generation highly concentrated low-volume product is available for adalimumab. In this product, the buffering and tonicity-adjustment agents used in the initial formulation have been eliminated to achieve a buffer-free solution that is stabilized essentially by using mannitol and polysorbate 80.
Although different life-cycle–management strategies have been adopted for several commercial biotherapeutics, the general approach would be to choose rationally an initial formulation and excipients with key considerations for life-cycle management as well. When formulations and excipients are not selected rationally, significant workarounds and delays can be expected for second-generation formulation and product development.
Taking a Careful, Deliberate Approach
The choice of sugars for biotherapeutic stabilization depends on a number of factors such as structure and stability of the biotherapeutic molecule, composition of its formulation, dosage-form type, and availability of highly pure excipients. Understanding the interplay of physicochemical properties and excipient quality, pharmaceutical processing conditions, and susceptibility of a biomolecule is important to delivering a stable biotherapeutic formulation. Although this review summarizes the landscape of biotherapeutic stabilization by sugars and polyols while providing a strategy for rational selection of sugar stabilizers, each biotherapeutic is unique. Stabilizer selection should be performed carefully in each case.
Concerns in Sugar Selection |
|---|
To discuss the different areas of concern regarding sugar excipient selection, consider the following questions: Are the structure, stability, and instability mechanisms of a biotherapeutic well known and characterized? Have stability studies been performed, or are they needed? Is the (native-state) stabilization mechanism well characterized? Do we understand the interplay between physicochemical properties of an excipient, the biomolecule’s structure and instability, and pharmaceutical processing conditions? How much stabilizer is enough? Is more sugar always good? Does the sugar excipient undergo changes during processing (e.g., polymorphism, crystallization)? Is the sugar excipient stable during all processing conditions (e.g., formulation pH, freeze–thaw, and room-temperature storage)? What is the dosage form for the biotherapeutic? Is it for self-administration or to be administered by a healthcare professional? How many indications does it address? Are new indications and disease areas being explored as the product is developed and commercialized? For assessing manufacturability, is the formulation susceptible to changes and instability during expected manufacturing conditions (e.g., pH fluctuations during freezing and thawing)? Are all excipients easily available, and is the supply chain robust? For quality control (QC), are the excipients highly pure and manufactured using well-characterized processes? Can underlying quality attributes affect formulation stability? Is compendial product good enough? What is the formulation development strategy for product life-cycle management (e.g., formulation and dosage-form changes after commercialization)? What about development of self-administration devices? |
Acknowledgments
I am grateful to Chris Yonan, PhD (executive director of process development at Oncobiologics Inc. in Cranbury, NJ) and Trevor Calkins, PhD (vice president of research and development at Pfanstiehl in Waukegan, IL) for helpful discussion about and review of this article.
References
1 Gokarn YR, et al. Excipients for Protein Drugs. Excipient Development for Pharmaceutical, Biotechnology, and Drug Delivery Systems. Ashok K, Chaubal M, Eds. CRC Press: Boca Raton, FL, 2006; 291–332.
2 Chi EY. Excipients Used in Biotechnology Products. Pharmaceutical Excipients: Properties, Functionality, and Applications in Research and Industry. Koo OMY, Ed. Wiley: Hoboken, NJ, 2016; 145–198.
3 Kamerzell TJ, et al. Protein–Excipient Interactions: Mechanisms and Biophysical Characterization Applied to Protein Formulation Development. Adv. Drug Deliv. Rev. 63, 2011: 1118–1159.
4 Nema S, Brendel J. Excipients and Their Role in Approved Injectable Products: Current Usage and Future Directions. PDA J. Pharm. Sci. Tech. 65, 2011: 287–332.
5 Ohtake S, Kita Y, Arakawa T. Interactions of Formulation Excipients with Proteins in Solution and in the Dried State. Adv. Drug Deliv. Rev. 63, 2011: 1053–1073.
6 Ohtake S, Wang YJ. Trehalose: Current Use and Future Applications. J. Pharm. Sci. 100, 2011: 2020–2053.
7 Wang W. Instability, Stabilization, and Formulation of Liquid Protein Pharmaceuticals. Int. J. Pharm. 185, 1999: 129–188.
8 Wang YJ, Hanson MA. Parenteral Formulations of Proteins and Peptides: Stability and Stabilizers. J. Parent. Sci. Technol. Technol. Rep. 10(42) 1988: S42.
9 Wang W, et al. Antibody Structure, Instability, and Formulation. J. Pharm. Sci. 96(1) 2007: 1–26.
10 Arakawa T, Timasheff SN. The Stabilization of Proteins By Osmolytes. Biophys. J. 47, 1985: 411–414.
11 Arakawa T, Timasheff SN. Preferential Interactions of Proteins with Solvent Components in Aqueous Amino Acid Solutions. Arch. Biochem. Biophys. 224, 1983: 169–177.
12 Gekko K, Timasheff SN. Mechanism of Protein Stabilization By Glycerol: Preferential Hydration in Glycerol–Water Mixtures. Biochem. 20, 1981: 4667–4676.
13 Lee JC, Timasheff SN. The Stabilization of Proteins By Sucrose. J. Biol. Chem. 256, 1981: 7193–7201.
14 Arakawa T, Timasheff SN. Stabilization of Protein Structure By Sugars. Biochem. 21, 1982: 6536–6544.
15 Nicoud L, et al. Effect of Polyol Sugars on the Stabilization of Monoclonal Antibodies. Biophys. Chem. 197, February 2015: 40–46.
16 Kaushik JK, Bhat R. Why Is Trehalose an Exceptional Protein Stabilizer? An Analysis of the Thermal Stability of Proteins in the Presence of the Compatible Osmolyte Trehalose. J. Biolog. Chem. 278, 2003: 26458–26465.
17 Cleland JL, et al. A Specific Molar Ratio of Stabilizer to Protein Is Required for Storage Stability of a Lyophilized Monoclonal Antibody. J. Pharm. Sci. 90(3) 2001: 310–321.
18 Prescribing Information: Herceptin (trastuzumab). Genentech: South San Francisco, CA, 2018: http://www.gene.com/download/pdf/herceptin_prescribing.pdf.
19 Carpenter JF, et al. Rationale Design of Stable Lyophilized Protein Formulations: Theory and Practice. Kluwer Academic/Plenum Publishers: New York, NY, 2002: 109–133.
20 Laursen T, Hansen B, Fisker S. Pain Perception After Subcutaneous Injections of Media Containing Different Buffers. Basic Clin. Pharmacol. Toxicol. 98(2) 2006: 218–221.
21 Yu AW, et al. Pain Perception Following Subcutaneous Injections of Citrate-Buffered and Phosphate-Buffered Epoetin Alpha. Int. J. Artif. Organs 21(6) 1998: 341–343.
22 Kappelgaard AM, et al. Liquid Growth Hormone: Preservatives and Buffers. Horm. Res. 62 (3) 2004: S98–S103.
23 Shire SJ, Shahrokh Z, Liu J. Challenges in the Development of High Protein Concentration Formulations. J. Pharm. Sci. 93(6) 2004: 1390–1402.
24 Safety Data Sheet: Herceptin Vials 60. F. Hoffmann-La Roche AG: Basel, Switzerland, 30 December 2017; www.roche-australia.com/content/dam/roche_australia/en_AU/files/SDS/HERCEPTIN_sds.pdf
25 Barnett GV, et al. Osmolyte Effects on Monoclonal Antibody Stability and Concentration-Dependent Protein Interactions with Water and Common Osmolytes. J. Phys. Chem. B 120(13) 2016: 3318–3330.
26 Chang L, et al. Mechanism of Protein Stabilization By Sugars During Freeze-Drying and Storage: Native Structure Preservation, Specific Interaction, and/or Immobilization in a Glassy Matrix? J. Pharm. Sci. 94(7) 2005: 1427–1444.
27 Han Y, et al. Effects of Sugar Additives on Protein Stability of Recombinant Human Serum Albumin During Lyophilization and Storage. Arch. Pharm. Res. 30, 2007: 1124.
28 Jovanović N, et al. Distinct Effects of Sucrose and Trehalose on Protein Stability During Supercritical Fluid Drying and Freeze-Drying. Eur. J. Pharm. Sci. 27(4) 2006: 336–345.
29 Duddu SP, Dal Monte PR. Effect of Glass Transition Temperature on the Stability of Lyophilized Formulations Containing a Chimeric Therapeutic Monoclonal Antibody. Pharm. Res. 14(5) 1997: 591–595.
30 Bhatnagar BS, Bogner RH, Pikal MJ. Protein Stability During Freezing: Separation of Stresses and Mechanisms of Protein Stabilization. Pharm. Dev. Technol. 12(5) 2007: 505–523.
31 Mensink MA, et al. How Sugars Protect Proteins in the Solid State and During Drying (Review): Mechanisms of Stabilization in Relation to Stress Conditions. Eur. J. Pharm. Biopharm. 114, May 2017: 288–295.
32 Cicerone MT, Pikal MJ, Qian KK. Stabilization of Proteins in Solid Form. Adv. Drug Deliv. Rev. 93, October 2015: 14–24.
33 He F, et al. High-Throughput Biophysical Approaches to Therapeutic Protein Development. Biophysics for the Life Sciences 4: Biophysics for Therapeutic Protein Development. Narhi LO, Ed. Springer Science Business Media: New York, NY, 2013.
34 Niedziela-Majka A, et al. High-Throughput Screening of Formulations to Optimize the Thermal Stability of a Therapeutic Monoclonal Antibody. J. Biomol. Screen. 20(4) 2015: 552–559.
35 Menzen T, Friess W. High-Throughput Melting-Temperature Analysis of a Monoclonal Antibody By Differential Scanning Fluorimetry in the Presence of Surfactants. J. Pharm. Sci. 102(2) 2013: 415–428.
36 Goldberg DS, et al. Formulation Development of Therapeutic Monoclonal Antibodies Using High-Throughput Fluorescence and Static Light Scattering Techniques: Role of Conformational and Colloidal Stability. J. Pharm. Sci. 100(4) 2011: 1306–1315.
37 He F, et al. High Throughput Thermostability Screening of Monoclonal Antibody Formulations. J. Pharm. Sci. 99(4) 2010: 1707–1720.
38 He F, et al. Screening of Monoclonal Antibody Formulations Based on High-Throughput Thermostability and Viscosity Measurements: Design of Experiment and Statistical Analysis. J. Pharm. Sci. 100(4) 2011: 1330–1340.
39 Samra HS, He F. Advancements in High Throughput Biophysical Technologies: Applications for Characterization and Screening During Early Formulation Development of Monoclonal Antibodies. Mol. Pharm. 9(4) 2012: 696–707.
40 Brader ML, et al. Examination of Thermal Unfolding and Aggregation Profiles of a Series of Developable Therapeutic Monoclonal Antibodies. Mol. Pharm. 12(4) 2015: 1005–1017.
41 Alekseychyk L, et al. High-Throughput Screening and Stability Optimization of Anti-Streptavidin IgG1 and IgG2 Formulations. J. Biomol. Screen. 19(9) 2014: 1290–1301; doi:10.1177/1087057114542431.
42 Razinkov VI, Treuheit MJ, Becker GW. Accelerated Formulation Development of Monoclonal Antibodies (MAbs) and MAb-Based Modalities: Review of Methods and Tools. J. Biomol. Screen. 20(4) 2015: 468–483.
43 Kang J, Lin X, Penera J. Rapid Formulation Development for Monoclonal Antibodies. BioProcess Int. 14(4) 2016: 40–44.
44 Warne NW. Development of High Concentration Protein Biopharmaceuticals: The Use of Platform Approaches in Formulation Development. Eur. J. Pharm. Biopharm. 78, 2011: 208–212.
45 Siedler M. Implementation of a Platform Approach for Early Biologics Development. Am. Pharm. Rev. 14(6) 2011.
46 Cavatur RK, et al. Crystallization Behavior of Mannitol in Frozen Aqueous Solutions. Pharm. Res. 19(6) 2002: 894–900.
47 Liao X, Krishnamurthy R, Suryanarayanan R. Influence of the Active Pharmaceutical Ingredient Concentration on the Physical State of Mannitol: Implications in Freeze-Drying. Pharm. Res. 22(11) 2005: 1978–1985.
48 Telang C, Suryanarayanan R, Yu L. Crystallization of d-Mannitol in Binary Mixtures with NaCl: Phase Diagram and Polymorphism. Pharm. Res. 20(12) 2003: 1939–1945.
49 Liao X, Krishnamurthy R, Suryanarayanan R. Influence of Processing Conditions on the Physical State of Mannitol: Implications in Freeze-Drying. Pharm. Res. 24(2) 2007: 370–376.
50 Pyne A, Surana R, Suryanarayanan R. Crystallization of Mannitol Below Tg‘ During Freeze-Drying in Binary and Ternary Aqueous Systems. Pharm. Res. 19(6) 2002: 901–908.
51 Telang C, Yu L, Suryanarayanan R. Effective Inhibition of Mannitol Crystallization in Frozen Solutions By Sodium Chloride. Pharm. Res. 20(4) 2003: 660–667.
52 Nunes C, et al. Characterization and Crystal Structure of d-Mannitol Hemihydrate. J. Pharm. Sci. 93(11) 2004: 2800–2809.
53 Hawe A, Friess W. Impact of Freezing Procedure and Annealing on the Physico-Chemical Properties and the Formation of Mannitol Hydrate in Mannitol–Sucrose–NaCl Formulations. Eur. J. Pharm. Biopharm. 64(3) 2006: 316–325.
54 Johnson RE, Kirchhoff CF, Gaud HT. Mannitol–Sucrose Mixtures: Versatile Formulations for Protein Lyophilization. J. Pharm. Sci. 91(4) 2002: 914–922.
55 Sek DC, Kin Ho. High Protein Concentration Formulations Containing Mannitol. US PTO Application 20080139792, 12 June 2008; https://patents.google.com/patent/US20080139792.
56 Sek DC, et al. Use of Sucrose to Suppress Mannitol-Induced Protein Aggregation. US PTO Application 20080200656, 21 August 2008; https://patents.google.com/patent/US20080200656A1/en.
57 Wakankar AA, et al. The Effect of Cosolutes on the Isomerization of Aspartic Acid Residues and Conformational Stability in a Monoclonal Antibody. J. Pharm. Sci. 96(7) 2007: 1708–1718.
58 Siew A. Freeze Drying Protein Formulations: Common Challenges and Key Considerations When Developing a Freeze-Drying Cycle for Protein Pharmaceuticals. Pharm. Technol. 38(5) 2014.
59 Bhatnagar B. Frozen Liquid vs. Freeze Dried Cake: Better Approach for First-in-Human Clinical Studies? AAPS National Biotechnology Conference, 18 May 2016.
60 Kolhe P, Amend E, Singh SK. Impact of Freezing on pH of Buffered Solutions and Consequences for Monoclonal Antibody Aggregation. Biotechnol. Prog. 26(3) 2010: 727–733.
61 Miller MA, et al. Frozen-State Storage Stability of a Monoclonal Antibody: Aggregation Is Impacted By Freezing Rate and Solute Distribution. J. Pharm. Sci. 102(4) 2013: 1194–1208.
62 Kueltzo LA, et al. Effects of Solution Conditions, Processing Parameters, and Container Materials on Aggregation of a Monoclonal Antibody During Freeze–Thawing. J. Pharm. Sci. 97(5) 2008: 1801–1812.
63 Singh SK, et al. Large-Scale Freezing of Biologics — A Practitioner’s Review, Part One: Fundamental Aspects. BioProcess Int. 7(10) 2009: 32–44.
64 Singh SK, et al. Large-Scale Freezing of Biologics — A Practitioner’s Review, Part Two: Practical Advice. BioProcess Int. 7(11) 2009: 34–42.
65 Singh SK. Storage Considerations As Part of the Formulation Development Program for Biologics. Am. Pharma. Rev. 10(3) 2007: 26–33.
66 Rayfield WJ, et al. Impact of Freeze/Thaw Process on Drug Substance Storage of Therapeutics. J. Pharm. Sci. 24 March 2017; doi:10.1016/j.xphs.2017.03.019.
67 Singh SK, et al. Large-Scale Freezing of Biologics: Understanding Protein and Solute Concentration Changes in a Cryovessel — Part 1. BioPharm Int. 23(6) 2010: 53–60.
68 Singh SK, et al. Large-Scale Freezing of Biologics: Understanding Protein and Solute Concentration Changes in a Cryovessel — Part 2. BioPharm Int. 23(7) 2010: 40–49.
69 Puri M, et al. Evaluating Freeze–Thaw Processes in Biopharmaceutical Development: Small-Scale Study Designs Evaluating Freeze–Thaw Processes in Biopharmaceutical Development. BioProcess Int. 13(1) 2015: 34–45.
70 Kolhe P, Badkar A. Protein and Solute Distribution in Drug Substance Containers During Frozen Storage and Post-Thawing: A Tool to Understand and Define Freezing-Thawing Parameters in Biotechnology Process Development. Biotechnol. Prog. 27(2) 2011: 494–504.
71 Rodrigues MA, et al. Effect of Freezing Rate and Dendritic Ice Formation on Concentration Profiles of Proteins Frozen in Cylindrical Vessels. J. Pharm. Sci. 100(4) 2011: 1316–1329.
72 Connolly BD, et al. Protein Aggregation in Frozen Trehalose Formulations: Effects of Composition, Cooling Rate, and Storage Temperature. J. Pharm. Sci. 104(12) 2015: 4170–4184.
73 Singh SK, et al. Frozen State Storage Instability of a Monoclonal Antibody: Aggregation As a Consequence of Trehalose Crystallization and Protein Unfolding. Pharm. Res. 28(4) 2011: 873–885.
74 Demeule B, et al. New Methods Allowing the Detection of Protein Aggregates: A Case Study on Trastuzumab. MAbs 1(2) 2009: 142–150.
75 Arvinte T, et al. Aggregation of Biopharmaceuticals in Human Plasma and Human Serum: Implications for Drug Research and Development. MAbs 5(3) 2013: 491–500.
76 Luo S, Zhang B. Dextrose-Mediated Aggregation of Therapeutic Monoclonal Antibodies in Human Plasma: Implication of Isoelectric Precipitation of Complement Proteins. MAbs 7(6) 2015: 1094–1103.
77 Fischer S, Hoernschemeyer J, Mahler HC. Glycation During Storage and Administration of Monoclonal Antibody Formulations. Eur. J. Pharm. Biopharm. 70(1) 2008: 42–50.
78 Gadgil HS, et al. The LC/MS Analysis of Glycation of IgG Molecules in Sucrose Containing Formulations. J. Pharm. Sci. 96(10) 2007: 2607–2621.
79 Weinbuch D, et al. Nanoparticulate Impurities in Pharmaceutical-Grade Sugars and Their Interference with Light Scattering–Based Analysis of Protein Formulations. Pharm. Res. 32(7) 2015: 2419–2427.
80 Carpenter J, et al. Meeting Report on Protein Particles and Immunogenicity of Therapeutic Proteins: Filling in the Gaps in Risk Evaluation and Mitigation. Biologicals 38(5) 2010: 602–611.
81 Wang W, Ignatius AA, Thakkar SV. Impact of Residual Impurities and Contaminants on Protein Stability. J. Pharm. Sci. 103(5) 2014: 1315–1330.
82 Yan B, Boyd D. Breaking the Light and Heavy Chain Linkage of Human Immunoglobulin G1 (IgG1) By Radical Reactions. J. Biol. Chem. 286(28) 2011: 24674–24684.
83 Li G, et al. Elemental Impurities in Pharmaceutical Excipients. J. Pharm. Sci. 104(12) 2015: 4197–4206.
84 Stoner MR, et al. Protein–Solute Interactions Affect the Outcome of Ultrafiltration/Diafiltration Operations. J. Pharm. Sci. 93(9) 2004: 2332–2342.
85 Bolton GR, et al. Effect of Protein and Solution Properties on the Donnan Effect During the Ultrafiltration of Proteins. Biotechnol. Prog. 27(1) 2011: 140–152.
86 Teeters M, et al. Predicting Diafiltration Solution Compositions for Final Ultrafiltration/Diafiltration Steps of Monoclonal Antibodies. Biotechnol. Bioeng. 108(6) 2011: 1338–1346.
87 Miao F, et al. Theoretical Analysis of Excipient Concentrations During the Final Ultrafiltration/Diafiltration Step of Therapeutic Antibody. Biotechnol. Prog. 25(4) 2009: 964–972.
Hiten Gutka, PhD, is associate director of formulation development at Oncobiologics Inc., 7 Clarke Drive, Cranbury, NJ 08512; 1-609-619-3990; [email protected].
You May Also Like






