September 2014
As if manufacturing of investigational medicinal products (IMPs) weren’t challenging enough already, the appropriate storage and distribution of sensitive biological products can be an adventurous journey itself — especially if not carefully planned and managed. No one solution fits all situations. Many things must be evaluated during planning stages. For example, what is more important: time to delivery or quality of transport and product integrity until drug can be administered to a patient?
It is important to understand key storage and distribution strategies such as central depots, direct-to-site shipments, and mixed alternatives that combine central and local depots. There is no one-size-fits-all solution. Therefore, it is important to evaluate the pros and cons of each option and identify the optimal individual study approach for your product. Storage typically does not end at a depot, so you must ensure that materials are stored safely at clinical sites and even with patients who are participating ...
https://bioprocessintl.com/wp-content/uploads/2014/09/062014-SupplyChainChallenges.mp3
Global manufacturing of biopharmaceuticals for human use helps save the lives of millions of people and is a large commitment to public health. The industry operates in an environment with financial uncertainties and complex international supply chains, so the question of risk mitigation is paramount. There is an expectation that comprehensive risk mitigation programs should be in place to minimize the risk of supply chain interruptions that would negatively affect the manufacture of these vital therapeutics. Here we share how a natural disaster affected GE Healthcare Life Sciences’ supply chain and discuss how such a comprehensive risk mitigation program enabled proactive management of the crisis. Finally, we show how supply was maintained to support continued manufacturing of life-preserving therapeutics.
The Complexity of Manufacturing
Biopharmaceuticals are complex molecules. So the manufacture of consistent, high-q...
The audio from the following presentations is available in the
BPI Theater section
of our website.
ANTIBODY–DRUG CONJUGATES
Optimized Solutions for Better ADC Design Through SMARTag Technology
Gregory T. Bleck, global head of biologics R&D at Catalent Pharma Solutions
Bleck began by discussing the limitations of firstgeneration antibody–drug conjugate (ADC) technology:
Then he described Specific Modifiable Aldehyde Recombinant Tag (SMARTag) technology and illustrated how it addresses those issues. To maximize ADC performance, developers can use this patented aldehyde-tagging method for site-specific, programmable drug placement on monoclonal antibody (MAb) carriers. ADC product consistency is improved for better regulatory compliance. A library of proprietary cytotoxin linkers and conjugation chemistry are designed to provide stability and optimize efficacy. An efficient and scalable manufacturing process allows for one-step in vivo tag generation that is compatible with any cell-based expression system...
Bayer Healthcare
The fed-batch culture of Chinese hamster ovary (CHO) cells has become well established as the primary method of manufacturing therapeutic recombinant protein products for various disease indications. Fed-batch process-development approaches focus on supporting high–cell-density cultures that are crucial to achieving high product titers but lead to proportionately high nutritional demands. Exhaustion of key nutrients negatively affects cell growth and ability to produce recombinant proteins. To counter that problem, concentrated feeds are added to the culture. Such feeds tend to be difficult to prepare because of limitations in solubility. Often, high or low pH conditions and/or heating steps must be used to dissolve feed components. In many cases, the resulting solutions have a relatively short shelf life (
1
,
2
).
We at Bayer HealthCare implemented a novel, proprietary nutrient additive called GIBCO FunctionMAX TiterEnhancer (Life Technologies, now Thermo Fisher Scientific) in combinat...
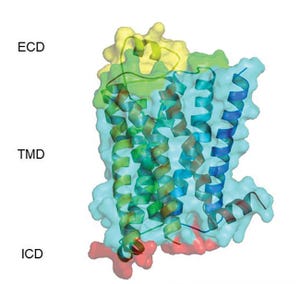
β
1
AR Crystal structure
depicting ECL2 in yellow
In part 1, we summarized the advances made in new approaches developed to address the challenges of antigen generation for targeting G protein–coupled receptors (GPCRs). We reviewed the antibody and biologics pipeline with progress highlighted by some interesting case studies on new targets (
1
). Here, we conclude by reviewing progress attained with other biologics.
Peptides Targeting G Protein–Coupled Receptors
More than 50 peptide-based therapeutic products are commercially available, but very few of them have been derived from recombinant display technology. In fact, only three peptide therapeutics targeting GPCRs have been discovered in that manner — one of which is ecallantide. Most others are either the endogenous agonist or a closely related analogue. However, in silico platforms also have been used for computational screenings. They are based on algorithms, such as Compugen’s peptide ligand discovery platform (
2
), which has identified potential ...
No longer are scientists bound to the time-consuming, error-prone use of dilution factors and fixed-pathlength measurements in determining the concentration of an analyte in solution. Using the slope spectroscopy technique, the Solo VPE system (from C Technologies) offers a new method of determining analyte concentration based on the Beer–Lambert law and slope derived from absorbance measurements made at multiple pathlengths (
1
).
Mathematics:
The Beer–Lambert law is expressed as
A = αlc
, where A is the measured absorbance, α is the molar absorption coefficient, l is the pathlength, and c is the sample concentration. This equation can then be rearranged for use with slope spectroscopy:
A/l = αc
. For measurements comparing slope and pathlength, a linear regression equation is written as
A = ml + b
, where m is the slope of the regression line, and b is the y-intercept. Dimensional equality then allows for replacement of the left-hand side of the second equation above with the slope term from the thi...
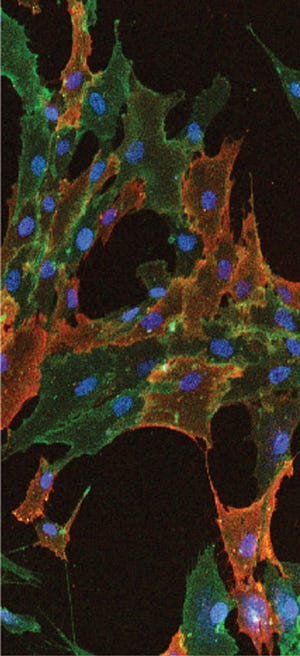
Immunocytochemical staining shows cultured human bone marrow–derived mesenchymal stem cells stained with STRO-1 and CD90 antibodies. Nuclei of the cells are
visualized using DAPI (blue).
EMD MILLIPORE
Cell therapy holds the promise of delivering the next generation of future medical breakthroughs. In this respect, multipotent progenitor cells such as human mesenchymal stem cells (hMSCs) have attracted high clinical interest because of their ability to differentiate into various cell types and their immunoregulatory properties. Furthermore, hMSCs express only low levels of class I major histocompatibility complex (MHC I) molecules on their surfaces and are therefore invisible to a host’s immune system. Finally, hMSCs can actively suppress the innate immune system by expressing a number of secreted factors such as factor H and human leukocyte antigen G5 (HLA-G5).
Together, those features enable the allogeneic use of hMSCs and thus make them an attractive target for commercial therapeutic development. Alloge...
Incubator holding 800–1,000 seeded trays
can produce ~1 kg crude protein overnight
MicroProtein Technologies Inc. has developed the MPTxpress high-yield, low‑cost, recombinant
Escherichia coli
manufacturing platform. Rather than using liquid culture media within stirred bioreactors, the system uses trays filled with semisolid (gelled) culture media overlaid with or without a permeable membrane on which the
E. coli
is cultured. Compared with conventional liquid fermentation platforms, the MPTxpress system reduces the number of steps in up- and downstream processing and required infrastructure, significantly improves yields, and lowers costs. It provides simplicity for mixing and upstream bioprocessing free of connections, tubing, and piping. The first commercial products being developed using this platform are protein A resins with improved acid and base stability and the ability to be recycled (e.g., over 100 times).
MicroProtein is the only company working to produce recombinant biologics at commerci...
https://bioprocessintl.com/wp-content/uploads/2014/09/092014_Locwin.mp3
For decades, professional biology education programs, universities, community colleges, and some high schools have inoculated students with the phrase central dogma to refer to the basic paradigm that “DNA encodes RNA, which encodes for protein” (
1
). Although that is in large part true, we do need to break with tradition, let science take its course, and call it like it is.
Background
In 1970, Francis Crick’s seminal paper in Nature (“Central Dogma of Molecular Biology”) was published. Eventually its premise was swept up in the imagination of the nation and the world (
2
). In that paper, Crick erroneously used the phrase central dogma to refer to this oft-seen relationship between deoxyribonucleic acid (DNA), ribonucleic acid (RNA), and proteins. Without getting caught up in the nuances and advances we’ve elucidated about the nature of protein coding since 1970, we need to move our field forward and change the formative education o...
https://bioprocessintl.com/wp-content/uploads/2014/09/082014_Langer-SingleUse.mp3
Pall KleenPak connector (www.pall.com)
Single-use equipment vendors are failing to meet their biomanufacturing customers’ expectations in several key areas, according to BioPlan Associates’ 11th Annual Report and Survey of Biopharmaceutical Manufacturing Capacity and Production (
1
). The study — based on a global survey of 238 global biomanufacturers and contract manufacturing organizations, as well as 158 vendors and suppliers in 30 countries — identifies significant gaps between the perceived importance of key vendor attributes and companies’ satisfaction with their vendors regarding those attributes.
One area in which users report the lowest degree of satisfaction is device
FIGURE 1: Selected percentage point gap between importance of single-use system product
attributes and level of satisfaction (selected data points from 11th Annual Report and Survey of
Biopharmaceutical Manufacturing)
Cost of product
standardization (...